| |
Abstract
Objective: To explore the in vitro antibacterial activity of ethanolic extracts of cinnamon (Cinnamomum zeylanicum; CIN), clove (Syzygium aromaticum, CLV) and cumin (Cuminum cyminum, CMN) against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA), from Kolkata, India.
Methods: The CIN, CLV and CMN were tested for their antibacterial activity against MRSA by in vitro methods. Minimum inhibitory concentration (MIC) values of the three extracts were determined, and time-kill studies were performed in order to investigate the bactericidal activity of the extracts (at the MIC level) for the isolates. The killing efficacy of the extracts was determined at various concentrations.
Results: The zone diameter of inhibition (ZDI) obtained due to CIN, CLV and CMN ranged between 22-27 mm, 19-23 mm and 9-15 mm, respectively; while the MICs, for the isolates, were in the range of 64-256, 64-512 and 128-512 µg/ml, respectively. When tested for their MIC levels; the CIN and CLV were found to be bactericidal after 6 hrs of incubation, while CMN showed bactericidal activity after 24 hrs. However, when tested at various concentrations; CIN, CLV and CMN displayed bactericidal activity against S. aureus, after 24 hrs of incubation, at 200, 200 and 300 µg/ml, respectively.
Conclusion: The C. zeylanicum and S. aromaticum showed the strongest in vitro antibacterial activity followed by C. cyminum against MRSA, and such findings could be considered a valuable support in the treatment of infection and may contribute to the development of potential antimicrobial agents for inclusion in anti- S. aureus regimens.
Keywords: MRSA; Zone diameter of inhibition; Minimum inhibitory concentration; Bactericidal activity; Spices.
Introduction
Antibiotic therapy in recent years has faced difficulties due to the rapid emergence of multidrug resistance among bacteria causing several life threatening infections, and this in turn, making the future management of infectious diseases uncertain. The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic microbial infectious agents has led to the screening of several medicinal plants for potential antimicrobial activity, and the plant extracts were found to have potential against microorganisms.1,2 The spices that are generally used as food additives in order to provide taste, smell, and color also exhibited antibacterial activity, as has been documented in the literature. Agaoglu et al.3 reported the antibacterial activity of different spices including Cinnamomum zeylanicum, Syzygium aromaticum and Cuminum cyminum, in terms of the zone diameter of inhibition (ZDI), against gram-negative and gram-positive bacteria including Staphylococcus aureus. Khan et al.4 reported that spices including C. zeylanicum and S. aromaticum can be used against multidrug resistant (MDR) microbes causing nosocomial and community acquired infections such as S. aureus. Nanasombat and Lohasupthawee reported inhibitory activity of C. zeylanicum, S. aromaticum and C. cyminum, based on the ZDI and minimum inhibitory concentration (MIC) values against various enteric bacteria.5
Antibacterial activities of some medicinal plants such as Althaea officinalis, Mentha longifolia, Melissa officinalis and Rosa damascene against methicillin-resistant S. aureus (MRSA) have been reported in terms of MICs.6 But the bactericidal activity of C. zeylanicum, S. aromaticum and C. cyminum has not been documented against S. aureus, which is implicated in diseases like wound infections, abscesses of the muscle, and urogenital tract infections. Moreover, the increasing emergence of nosocomial isolates of S. aureus exhibiting resistance not only to methicillin but also to other anti-S. aureus antibiotics,7,8 led to therapeutic problems, and hence searching the source for new, safe and effective alternative treatment regimen against MRSA infections is imperative in order to control its spread.
In the present study, time-kill experiments with the ethanolic extracts of the stem bark of cinnamon (Cinnamomum zeylanicum; CIN), flower bud and stalk of clove (Syzygium aromaticum, CLV) and seed of cumin (Cuminum cyminum, CMN), were carried out in order to determine their bactericidal effect on MRSA. The ZDI and MIC values of the test extracts for the isolates were also determined.
Methods
A total of 12 clinical isolates of S. aureus (7 from throat swab, 3 from pus and 2 from skin swab) showing resistance to methicillin by disk diffusion technique were considered for the present study, and the S. aureus ATCC 25923 strain was used as the control.
The Mueller-Hinton broth (MHB; for subculturing the strains, inocula preparation and performing time-kill experiments) and Mueller-Hinton agar (MHA; for the determination of ZDIs and MICs), obtained from Hi-Media (India), were used in the study. The bacterial inocula were prepared as 104 CFU/spot, 108 CFU/ml and 5 × 105 CFU/ml, respectively for agar dilution, agar diffusion and time-kill studies, following the previously described methodology.2
Three Indian spices (Table 1), in dried forms, were purchased from the local market in Kolkata, India, and used in the current study. The ethanolic extracts of the three spices were prepared following the published protocol,2 with slight modification. Briefly, 50 mg of each of the spices, after grinding into small granules or dusts, were soaked in 50 ml of 95% ethanol for 48 hrs, with vigorous shaking at 15 min interval initially for 6 hrs, followed by shaking at 2 hr intervals. Afterwards, the mixtures were filtered, and the filtrates were vaporized to dryness, and weighed in order to determine the % yield of the extracts, following the formula: % yield = (weight of extract / weight of ground plant material) × 100.9 The stock solutions of the crude ethanolic extracts were prepared by diluting the dried extracts: CIN, CLV and CMN, with 50% ethanol to obtain the final concentration of 10 mg/ml for each.
Table 1: Details of the spices used in the study.
|
Spices
|
Family |
Local name |
Part used |
Abbreviation (ethanolic extract) |
|
Cumin (Cuminum cyminum)
|
Umbelliferae |
Jeera |
Fruit |
CMN |
|
Clove (Syzygium aromaticum)
|
Myrtaceae |
Labanga |
Flower stalk and bud |
CLV |
|
Cinnamon (Cinnamomum zeylanicum)
|
Lauraceae |
Darchini |
Stem bark |
CIN |
Agar diffusion technique, as described by Nas,10 was followed in order to determine the ZDIs, using an inoculum of 108 CFU spread on MHA plates. Each of the inoculated plates was divided into four equal sectors, and the extracts, 20 µl (equivalent to 200 µg) each, were dropped onto the sectors marked previously with CIN, CLV and CMN; onto the final sector 20 µl of 50% ethanol was used for control. The plates were then incubated at 350C for 24 hrs, and after that period, ZDIs produced due to the action of the extracts were recorded for all the isolates. The control sectors results no ZDI to ZDI of 6 mm for the test bacterial isolates, and therefore, the sensitivity to the plant extracts for the MRSA isolates were considered with ZDI ≥7 mm, which has also been considered by earlier authors.1,11
The determination of MICs of the extracts was conducted following agar dilution technique, using 104 CFU /spot as the inoculum and the concentrations of the extracts used were 512, 256, 128, 64 and 32 µg/ml; the other details are described elsewhere.2
Bacterial killing studies were conducted for the S. aureus TSB3 strain that had MIC 256 μg/ml for each of the extracts CMN, CLV and CIN, in MHB with an initial inoculum of approximately 5 × 105 CFU/ml, following the earlier published protocol.2 In this method, viable cell counts were determined at 0, 3, 6 and 24 hrs, in the presence of each of the extracts at concentration of 256 μg/ml (1 × MIC). To determine the effect of varied concentrations of the extracts (25-300 μg/ml) on bacterial density (CFU/ml), the bacterial suspension (5 × 105 CFU/ml) in MHB was incubated for 24 hrs at 350C. The ≥3 log10 decrease in the inoculum after 24 hrs of incubation was considered as the bactericidal activity.12
The X2-test was employed to compare the antibacterial activities of CIN, CLV and CMN, based on the bacterial growth (in terms of CFU/ml) at different concentrations and at different time intervals. A p value of <0.05 was considered significant.
Results
The agar dilution test results are represented in Fig. 1. All the S. aureus isolates were found to be sensitive to the crude ethanolic extracts of the three spices tested. The isolates had ZDI 22-27 mm, 19-23 mm and 9-15 mm against CIN, CLV and CMN, respectively.
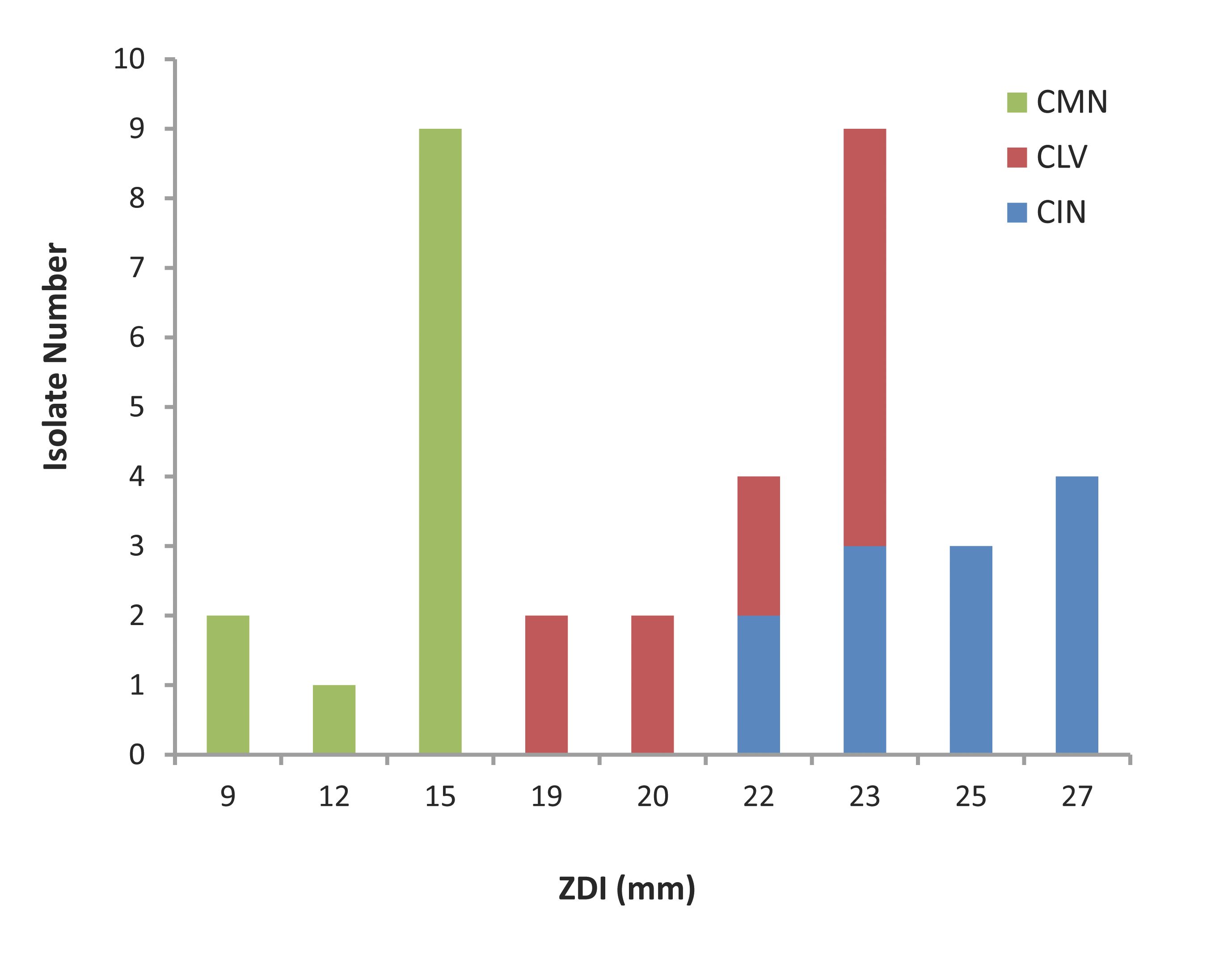
Figure 1: Zone diameter of inhibition (ZDI) of three spices for MRSA isolates. CIN= Cinnamomum zeylanicum; CLV= Syzygium aromaticum; CMN= Cuminum cyminum.
The MICs of CIN, CLV and CMN for the 12 S. aureus isolates (Fig. 2) ranged as follows; 64-256, 64-512 and 128-512 μg/ml, respectively. Most of the isolates; 8 (66.67%) and 9 (75%) had CIN and CMN MICs 256 μg/ml and 512 μg/ml, respectively, while 9 (75%) showed CLV MICs in between 256 μg/ml and 512 μg/ml. Moreover, 3 (25%) and 2 (16.67%) isolates had MIC of 64 μg/ml, respectively to CIN and CLV.
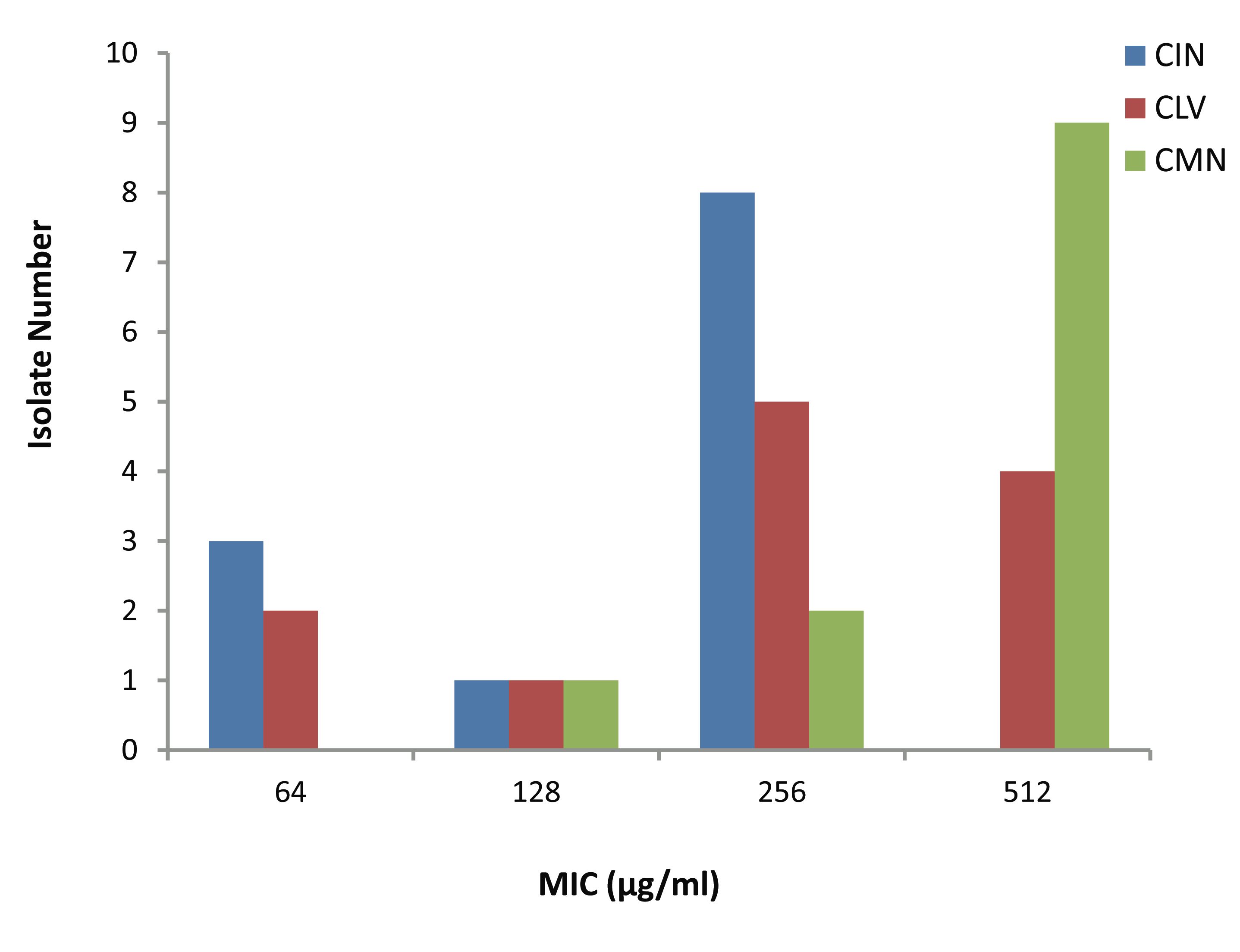
Figure 2: Minimum inhibitory concentration (MIC) of three spices for MRSA isolates. CIN= Cinnamomum zeylanicum, CLV= Syzygium aromaticum, CMN= Cuminum cyminum.
Fig. 3 depicts the killing activities of various concentrations (25-300 μg/ml) of CIN, CLV and CMN against S. aureus. At the lowest concentration (25 μg/ml), CIN, CLV and CMN did not affect the bacterial growth, and at this concentration the bacterial density increased up to 6.102, 6.3 and 6.254 log10 CFU/ml, respectively. The CIN and CLV at 50 μg/ml inhibited the growth of S. aureus, while the CMN began to show a growth inhibitory effect at 100 μg/ml. The CIN and CLV showed bactericidal activity at concentration 200 μg/ml against S. aureus reducing 4.508 log10 CFU/ml and 3.978 log10 CFU/ml, respectively; CMN exhibited bactericidal effects at 300 μg/ml, leaving 0.59 log10 CFU/ml, after 24 hrs.
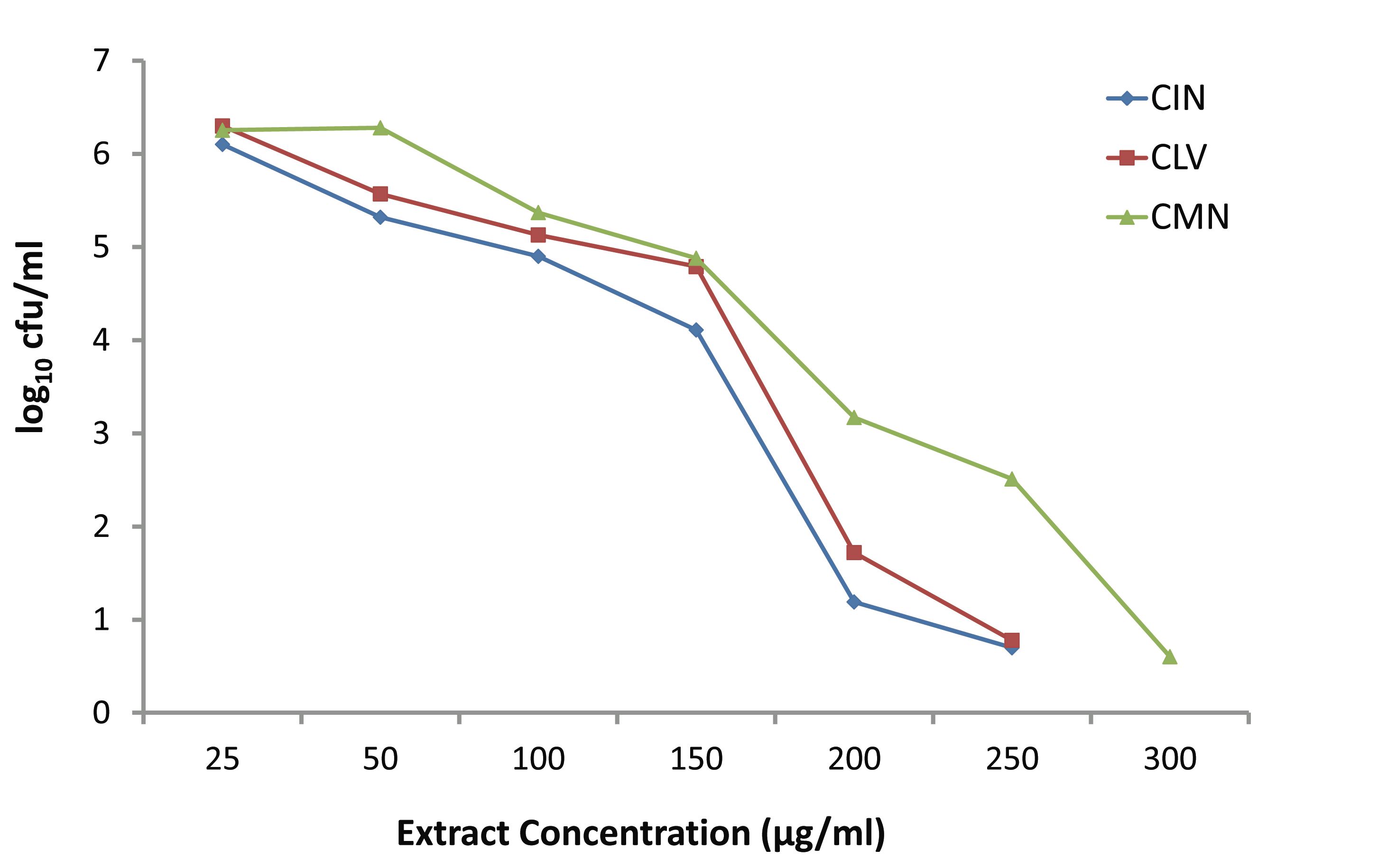
Figure 3: Effect of various concentrations of CIN (Cinnamomum zeylanicum), CLV (Syzygium aromaticum) and CMN (Cuminum cyminum) on growth of MRSA.
The time dependent killing effect of CIN, CLV and CMN at concentration 256 μg/ml (1 × MIC) on S. aureus strain is depicted in Fig. 4. In the presence of 256 μg/ml of CIN, CLV and CMN; the viable cell counts were decreased after 3 hrs by up to 3.27, 3.61 and 3.97 log10 CFU/ml, respectively. The CIN and CLV had bactericidal effects at 6 hrs, when the viable cell counts decreased to 2.12 and 2.39 log10 CFU/ml, respectively. However, the CMN showed bactericidal effect on S. aureus after 24 hrs of incubation, decreasing the viable cell count to 4.218 log10 CFU/ml.
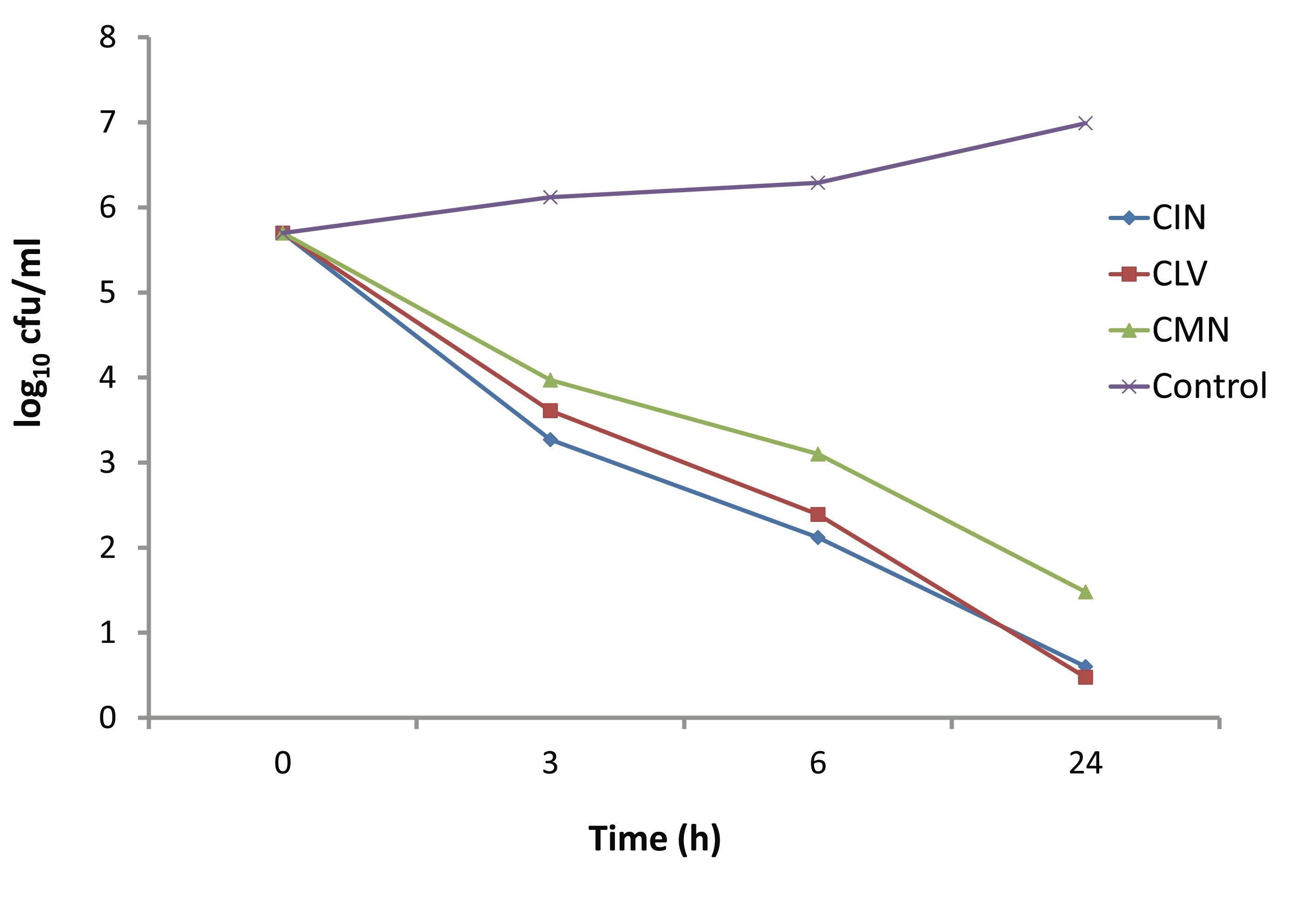
Figure 4: Kill kinetics of CIN (Cinnamomum zeylanicum), CLV (Syzygium aromaticum) and CMN (Cuminum cyminum) against MRSA.
The significant differences in killing were found between CIN and CLV (p<0.05), as well as between CIN and CMN (p<0.05), when 200-250 μg/ml concentrations (bactericidal concentrations) were considered, as has been found in the first set of killing studies, (Fig. 3). In the second set of time-kill experiments; CIN, CLV and CMN at a concentration of 256 μg/ml (1 × MIC) showed significant killing activities (p<0.05), both at 3 and 6 hrs. (Fig. 4)
Discussion
The antibacterial activity of C. zeylanicum, S. aromaticum and C. cyminum, in terms of ZDI, has been reported in earlier studies. Agaoglu et al.3 reported C. zeylanicum as the most effective spice against the microorganisms tested, and the most susceptible bacterial strain to this spice was S. aureus (ZDI: 32 mm); the ZDIs due to the action of S. aromaticum and C. cyminum against S. aureus were documented as 15 mm and 10 mm, respectively. Fabio et al.13 reported ZDIs of 24 and 18 mm, respectively for C. zeylanicum and S. aromaticum against S. aureus.
In the current study, the three ethanolic extracts (CIN, CLV and CMN) displayed excellent antibacterial activity against MRSA, since the all 12 (100%) isolates showed sensitivity to the extracts with ZDI >7 mm. The CIN exhibited the best anti-S. aureus activity producing ZDI ranging between 22 mm and 27 mm; most (n=7; 58.33%) of the isolates were highly sensitive to CIN (ZDI: 25-27 mm). By the agar diffusion method, the growth inhibitory activity was followed by the action of CLV with ZDI 19-23 mm; 8 (66.67%) were the most sensitive isolates for which the ZDI were 22-23 mm. The isolates showed relatively less sensitivity to CMN; however 9 (75%) of the tested isolates had ZDI 15 mm. Thus, the three spice-extracts exhibited different degrees of growth inhibition against S. aureus isolates, and the ranking of anti-S. aureus activity of the extracts tested is CIN>CLV>CMN.
Based on the above findings, the three extracts were subjected to further evaluation of their effects on MRSA by MIC determination following agar dilution method, and the study results supported the establishment of similar ranking (CIN>CLV>CMN) of antibacterial activity, as has been mentioned above. The CMN exhibited the highest MIC values; the 9 (75%) isolates had maximum MICs of 512 μg/ml. The isolates though had CLV MIC range of 64-512 μg/ml; only 2 (16.67%) had MIC of 512 μg/ml.
Agar dilution test results revealed that S. aureus were most susceptible to CIN (MICs: 64-256 μg/ml), which showed the highest antibacterial activity in terms of ZDI obtained for the isolates. Abu-Shanab reported antibacterial activity in terms of MICs on ethanolic extracts of M. longifolia (MICs: 3.125-12.5 mg/ml), M. officinalis (MICs: 3.125-12.5 mg/ml) and R. damascene (MICs: 0.3951-0.78 mg/ml) against MRSA.6 Nanasombat determined the MICs of ethanolic extracts of various spices including C. zeylanicum, S. aromaticum and C. cyminum for a number of gram-negative enteric bacteria; and found S. aromaticum to be the most effective spice (MICs: 2.6-41.7 mg/ml).5 However, the earlier investigators did not determine MICs of C. zeylanicum, S. aromaticum and C. cyminum extracts against S. aureus.3,13 Prabuseenivasan et al. reported that C. zeylanicum oil showed maximum activity with MICs ranging from 0.8 to 3.2 mg/ml, followed by S. aromaticum oil with MICs between 1.6 mg/ml and 6.4 mg/ml against bacterial isolates including S. aureus.14
The C. zeylanicum bark is rich in cinnamaldehyde (50.5%), which has been proven to be active against many pathogenic gram-positive and gram-negative bacteria.15,16 Ali et al. reported cinnamaldehyde as the active agent to inhibit the growth of both antibiotic-sensitive and -resistant stains of Helicobacter pylori.17 It has been reported that S. aromaticum oil contains high (75%) eugenol, and the antibacterial activity of S. aromaticum is attributed to this compound.18,19 Another important antimicrobial compound is tannin, in S. aromaticum, which also aids the process of antimicrobial action. The antibacterial activity of C. cyminum essential oil is perhaps attributable to the high levels of cumin aldehyde (16.1%), the other main component includes a-pinene (11.4%).20,21
Nanasombat and Lohasupthawee reported that carvone and carvacrol are also known to have inhibitory effects on bacterial strains.5 Here, we have not studied the antibacterial activity of the components within the extracts; however, the compounds mentioned above may be responsible for the anti-MRSA activity of the extracts, as has been investigated in our study, and the extracts exhibited broad-spectrum antibacterial activity against both gram-positive and gram negative strains.6
A system has been demonstrated earlier that shows the rate and extent of bacterial killing (kill kinetics) providing more accurate description of antimicrobial activity than the MIC does, and it has also displayed better sensitivity trends to physicians than disk diffusion methods.2,22 Okemo et al.23 investigated the rate and extent of bacterial killing with stem-bark extract of Azadirachta indica against S. aureus, and Mandal et al.2 reported the results of time-kill experiments with A. indica seed extract against Salmonella enterica serovar Typhi. In the present report, two sets of time-kill experiments were performed for the first time, at least in this part of the globe, with three spice-extracts CIN, CLV and CMN against MRSA, using 5 × 105 CFU/ml of the initial inoculum. When increasing concentrations (25-300 μg/ml) of the extracts were used, no significant difference in killing was found among the extracts up to the concentration of 150 μg/ml after 24 hrs. Moreover, in the presence of the lowest concentration (25 μg/ml) of CIN, CLV and CMN; bacterial population increased to 6.102, 6.3 and 6.254 log10 CFU/ml, respectively. Raising the concentration of the extracts increased the rate and amount of killing at 24 hrs; at the concentration of 200 μg/ml, CIN and CLV had bactericidal effect on MRSA, thus decreasing cell counts by 4.522 log10 CFU/ml and 4.252 log10 CFU/ml, respectively, and significant differences in killing were found between CIN and CLV (p<0.05), and between CIN and CMN (p<0.05).
The CMN exhibited bactericidal activity at a concentration of 300 μg/ml, while CIN and CLV, at this concentration, killed the whole bacterial population in 24 hrs. In another set of time-kill experiments; CIN, CLV and CMN at a concentration of 256 μg/ml (1×MIC) showed significant killing activity (p<0.05) at 3 hrs, reducing viable cell counts up to 3.27, 3.61 and 3.97 log10 CFU/ml, respectively, compared to the initial inocula, 5 × 105 CFU/ml (5.698 log10 CFU/ml). At 6 hrs, CIN and CLV (both at 256 μg/ml) showed bactericidal effects against MRSA, decreasing the bacterial population to 2.12 and 2.39 log10 CFU/ml, respectively; thus a significant difference in the viable cell counts (p<0.05) were observed between the extracts (CIN and CLV) as well as between CLV and CMN (that reduced the population to 3.1 log10 CFU/ml). The CMN (256 μg/ml) displayed bactericidal effect at 24 hrs with a reduction of bacterial population by 4.218 log10 CFU/ml. As a result, it is interesting to note that for S. aureus; the killing with CIN, CLV and CMN was both dosage and time dependent, indicating that antimicrobial substances in the tested spices might affect the synthesis of the peptidoglycan layer of the cell wall and the mode of action of the spice extracts is cell wall related.2,23 This in turn implies a more rational basis for determining the optimal dosage for antimicrobial treatment regimens,24 in order to combat the emergence and spread of antimicrobial resistance.
Conclusion
The degree of antibacterial activity of the spices tested can be represented in the following order: C. zeylanicum > S. aromaticum > C. cyminum; all the three spices were found to be excellent bactericidal agents. Thus, C. zeylanicum, S. aromaticum, C. cyminum could be selected for use as potential anti- MRSA agents, which will also be of great benefit in combating antibiotic resistance of S. aureus causing serious human infections as found in the current study. However, further studies are required to determine the toxicity and proper dose selection, but before that; these agents can easily be utilized topically.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
1. Mandal S, Mandal MD, Pal NK, Saha K. Synergistic anti-Staphylococcus aureus activity of amoxicillin in combination with Emblica officinalis and Nymphae odorata extracts. Asian Pacific J Trop Med 2010;3:711-714
2. Mandal S, Mandal MD, Pal NK. Antibacterial potential of Azadirachta indica seed and Bacopa monniera leaf extracts against multidrug resistant Salmonella enterica serovar Typhi isolates. Arch Med Sci 2007;3:14-18.
3. Agaoglu S, Dostbil N, Alemdar S. Antimicrobial activity of some spices used in the meat industry. Bull Vet Inst Pulawy 2007;51:53-57.
4. Khan R, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 2009;14(2):586-597.
5. Nanasombat S, Lohasupthawee P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against salmonellae and other enterobacteria. KMITL Sci Tech J 2005;5:527-538.
6. Abu-Shanab B, Adwan G, Jarrar N, Abu-Hijleh A, Adwan K. Antibacterial activity of four plant extracts used in Palestinee in folkloric medicine against methicillin-resistant Staphylococcus aureus. Turk J Biol 2006;30:195-198.
7. Schito GC. The importance of the development of antibiotic resistance in Staphylococcus aureus. Clin Microbiol Infect 2006 Mar;12(Suppl 1):3-8.
8. Korakaki E, Aligizakis A, Manoura A, Hatzidaki E, Saitakis E, Anatoliotaki M, et al. Methicillin-resistant Staphylococcus aureus osteomyelitis and septic arthritis in neonates: diagnosis and management. Jpn J Infect Dis 2007 May;60(2-3):129-131.
9. Banso A, Adeyemo S. Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemistri 2006;18:39-44.
10. Nas MN. In vitro studies on some natural beverages as botanical pesticides against Erwinia amylovora and Curtobacterium flaccumfaciensis subsp. Poinsettiae. Turk J Agric For 2004;28:57-61.
11. Nascimento GG, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol 2000;31:247-256 .
12. Leclercq R, Bingen E, Su QH, Lambert-Zechovski N, Courvalin P, Duval J. Effects of combinations of ß-lactams, daptomycin, gentamicin, and glycopeptides against glycopeptide-resistant enterococci. Antimicrob Agents Chemother 1991 Jan;35(1):92-98.
13. Fabio A, Cermelli C, Fabio G, Nicoletti P, Quaglio P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytother Res 2007 Apr;21(4):374-377.
14. Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 2006;6:39.
15. Gupta C, Garg AP, Uniyal RC, Kumari A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr J Microbiol Res 2008;9:247-251.
16. Oussalah M, Caillet S, Lacroix M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J Food Prot 2006 May;69(5):1046-1055.
17. Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, et al. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob 2005;4:20.
18. Lakshmi BS, Naidu KC. Antimicrobial efficacy of essential oil Syzygium aromaticum against common infectants of storage cereals and fruits. J Pharma Res 2010;3:2544-2545.
19. Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000 Feb;88(2):308-316.
20. Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, et al. Characterization of the action of selected essential oil components on gram-negative bacteria. J Agric Food Chem 1998;46:3590-3595 .
21. Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J Agric Food Chem 2005 Jan;53(1):57-61.
22. Mandal S. DebMandal M, Pal NK, Saha K. Inhibitory and killing activities of black tea (Camellia sinensis) extract against Salmonella enterica serovar Typhi and Vibrio cholerae O1 biotype El Tor serotype Ogawa isolates. Jundishapur J Microbiol. 2011;4:115-121.
23. Okemo PO, Mwatha WE, Chhabra SC, Fabry W. The kill kinetics of Azadirachta indica a. juss. (meliaceae) extracts on Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. African J Sci Technol 2001;2:113-118.
24. Chalkley LJ, Koornhof HJ. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob Agents Chemother 1985 Aug;28(2):331-342.
|
|