In March and April 2009, Mexico experienced outbreaks of an influenza-like illness. By 23 April 2009, several cases in Mexico and the United States (US) were confirmed to have been caused by a new swine-origin H1N1 influenza virus.1 The virus was spreading rapidly in Mexico and to different states of the US. On 25 April 2009, the World Health Organization (WHO) announced the situation to be “a public health emergency of international concern”.2 Soon, the virus spread to different countries in different continents. On 11 June 2009, the WHO announced the emergence of an influenza pandemic.3 By 14 February 2010, laboratory confirmed cases of pandemic H1N1 influenza were reported in more than 212 countries and overseas territories, with a death toll of at least 15 921.4
Influenza viruses belong to the family Orthomyxoviridae. These are enveloped viruses, with a segmented RNA genome. They are divided into three types: A, B, and C. Influenza A viruses are further divided into subtypes based on their main antigens: hemagglutinin (H) and neuraminidases (N). Immune response to these two antigens provides protection when the same virus is encountered again. Major changes in these two antigens (antigenic shift) produce a virus to which most of the population is susceptible, and hence, a pandemic can result. Pigs are considered “mixing vessels” for influenza virus. Apart from getting infected by porcine types of influenza virus, pigs can also get infected by avian types and human types. When different virus types infect one cell in a pig simultaneously, reassortment of the viral genome segments can happen resulting in a novel type of virus. If such virus is introduced into the human population with sustained human-human transmission, a pandemic occurs.5
In Oman, the first reported confirmed case of pandemic H1N1 influenza was in June 2009. By January 2010, 7 040 cases and 31 deaths were reported.6 Whether patients infected with pandemic H1N1 influenza behaved similarly to those infected with seasonal influenza was a point of major interest. This is why it was important that different medical institutions reported their experience with the virus. Few papers have come out from various hospitals in Oman to describe clinical and laboratory features of pandemic H1N1 influenza infection.7-9
We present the first description of clinical features, laboratory findings, and outcomes of pandemic H1N1-infected patients admitted to the Sultan Qaboos University Hospital (SQUH). SQUH is one of the main tertiary care hospitals in Oman.
Methods
This is a retrospective study on patients admitted with pandemic influenza A H1N1 during the first wave of the pandemic in Oman in SQUH. Subjects were admitted between August and December 2009. The study included both adult and pediatric patients.
We reviewed the clinical and laboratory data of H1N1-infected admitted patients from the hospital track-care computer system. All patients with positive ploymerase chain reaction (PCR) from respiratory samples during the five months period were included. These samples were tested for influenza A, influenza A pandemic subtype H1N1, and influenza B by real-time reverse-transcriptase–PCR (RT-PCR) using the Fast-Track Diagnostic kit (Fast-track Diagnostics, Junglinster, Luxembourg). Respiratory samples submitted for testing were mainly upper respiratory samples and included nasal/throat swabs, nasopharyngeal aspirates, and sputum samples. The samples were extracted manually using the Qiagen kit (QIAamp Viral RNA kit, GmbH, Hilden, Germany) and amplified using the Abbott m2000 real time system (Abbott Molecular, Wiesbaden, Germany). Epidemiological measures, clinical presentations, laboratory investigations, medications received, and outcome were all included in the data collection sheet. The severity criteria were adopted from the WHO categories and included mild, progressive, and severe [Table 1]. Ethical approval was obtained from the ethics committee at SQUH in 2009.
Table 1: Case severity definition in pandemic influenza, clinical management of human infection with pandemic (H1N1) 2009: revised guidance. World Health Organization, November 2009.10
|
Clinical features |
Influenza-like illness
Diarrhea with no signs of dehydration
No dyspnea |
Presenting clinical or radiological evidence of:
Lower respiratory tract infection.
CNS involvement.
Exacerbation of underlying chronic disease:
Asthma, COPD, bronchiectasis.
Congestive heart failure.
Hepatic or renal insufficiency.
Presentation with multi-organ failure of septic shock.
Severe dehydration. |
Uncomplicated presentation with progression of symptoms in the first 24 hours:
Worsening hypoxemia or dyspnea.
Signs of CNS complications like altered level of consciousness, confusion or seizures.
Evidence of sustained viral replication or invasive bacterial infection.
Severe dehydration. |
All frequency data on the clinical presentation, reasons for admission, comorbidities, and hematological abnormalities was recorded in Excel. The R software package (www.r-project.org) was used for chi-square analysis, which was done to investigate the strength of association between liver function tests and the category of severity. Multivariable analysis was used to study the relationship between the length of hospital stay following H1N1 admission and certain clinical and laboratory features. These variables were chosen using an extensive literature search.11-13 The generalized linear model, with Poisson distribution, was used for multivariable analysis. Coefficients of the model for each variable were calculated as [exp(βn)] and the 95% confidence interval (CI) levels were calculated using the formula exp (βn±1.96 × SE).
Results
During the study period, a total of 5109 patients were screened for influenza virus, 1388 were positive for influenza A H1N1 by PCR of whom 68 were admitted. Clinical and laboratory data were collected for the 68 admitted patients. This cohort included 41 (60.3%) females and 27 (39.7%) males. The average time between onset of illness and admission to the hospital was three days (1–14 days), and the average length of stay was also three days. The ages of the admitted patients ranged from 25 days to 67 years (median = 23 years). There were 31 patients (45.6%) < 18 years of age, and 11 (16.2%) patients were < 2 years old.
Patients presented with various clinical manifestations [Figure 1a]. Fever was the most common symptom and occurred in all the patients included in the study. Other clinical manifestations were cough (79.0%), rhinorrhea (50.0%), sore throat (31.0%), shortness of breath (25.0%), and myalgia (24.0%). Extrapulmonary symptoms such as vomiting and diarrhea were present in 25.0% and 15.0% of patients, respectively, in the adult and pediatric age groups combined. The study also looked at the reasons for admission to the hospital among the patients with H1N1 infection [Figure 1b]. The most common reason for admission was the severity of illness (69.1%) followed by the presence of comorbidities (61.7%). The most common comorbidities warranting admission were chronic lung disease and hematological diseases (13 and 12 patients, respectively) [Figure 1c]. Out of the 13 patients with chronic lung disease, 11 (84.6%) had asthma and out of the 12 patients with hematological diseases, nine (75.0%) had sickle cell disease. Five (7.4%) patients were pregnant.
The severity of illness of the admitted patients was classified based on WHO criteria. Sixteen patients (23.5%) had the uncomplicated disease, 31 (45.6%) had progressive disease, and 21 (30.9%) had a severe disease. Uncomplicated cases were admitted mainly due to the presence of comorbidities.
Chest X-ray was performed in 56 patients, 12 patients had lobar consolidation whereas 16 patients had bilateral consolidations. The rest were normal.
The laboratory findings of H1N1 infected inpatients in the study are summarized in Figure 1d. The most common hematological abnormalities were lymphopenia (41.8%), followed by neutropenia (20.9%). Other hematological abnormalities included anemia (17.9%), thrombocytopenia (16.4%), neutrophilia (14.9%), and lymphocytosis (6.0%). Liver function tests (LFTs) were measured in 42 patients. Of those, 57.1% had a rise in their alanine aminotransferase (ALT) and aspartate aminotransferase (AST). These results were interpreted according to the normal reference ranges used by the different laboratories at SQUH.

Figure 1: The (a) clinical presentation (b) reason for admission, (c) comorbidities, and (d) hematological abnormalities in patients admitted with influenza A H1N1 infection.
Regarding the treatment received, 66 (97.1%) received oseltamivir alone. One patient received oseltamivir and nebulized zanamivir. The median time from illness onset to initiation of treatment was 2.5 days (range = 1–14 days). Steroids were administered to 11 patients in our study; all had consolidation evident in their chest X-ray. Five patients were admitted to the intensive care unit (ICU) and required mechanical ventilation. One patient who was admitted to the ICU with acute respiratory distress syndrome died. This patient was 25 years old with no comorbidities and presented with shock and multi-organ failure after three days of febrile illness. He was started immediately on broad-spectrum antibiotics and oseltamivir. Nebulized zanamivir was added at a later stage; however, the patient died after 20 days in the ICU.
A multivariable analysis was done to investigate the relationship between the length of hospital stay and clinical and laboratory features [Table 2]. The sample size used for this analysis was 42 (excluding all patients without ALT results). As would be expected, we found that severe and progressive cases (combined) stayed longer in the hospital than non-severe (uncomplicated category) cases. Controlling for severity, we also found that the presence of anemia, lymphopenia, abnormal ALT, and the use of steroids independently increased the length of stay.
Table 2: Multivariable analysis of the length of hospital stay of H1N1 cases between September and December 2009.
|
Severe and progressive cases (by category) |
1.42 (1.06–1.91) |
|
Presence of comorbidities |
1.15 (0.86–1.57) |
|
Steroid use |
2.06 (1.55–2.73) |
|
Presence of anemia |
1.60 (1.11–2.27) |
|
Presence of lymphopenia |
1.72 (1.29–2.29) |
|
Presence of thrombocytopenia |
1.16 (0.87–1.53) |
|
Abnormal ALT |
1.83 (1.38–2.43) |
ALT: alanine amino transferase.
On classifying ALT, comorbidities, lymphocytes, and thrombocytes according to the category of severity, we found that there was no statistical association between the percentages [Figure 2].
Discussion
This is the first study demonstrating the epidemiological and clinical features of patients admitted to SQUH for pandemic influenza A H1N1 infection during the first wave in 2009. We used multivariable analysis to investigate the factors that influenced the length of hospital stay of these patients. During the five month period of our study, 68 patients were admitted of whom five required intensive care and mechanical ventilation. One patient died. Most patients were young; the median age was 23 years. Fever and cough were the main clinical manifestations, and 61.8% of patients had comorbidities predisposing them to severe infection leading to hospital admission.
All patients were treated with oseltamivir with variability in when treatment was started relative to the illness’s onset. Patient’s hospital stay was prolonged in severe cases, and in the presence of anemia, lymphopenia, and abnormal ALT. The use of steroids also independently increased the length of stay. The most common clinical manifestation observed in our cohort was fever. Similarly, fever was the main symptom reported in hospitalized patients in other hospitals in Oman (85–95%) and other countries such as in the United Kingdom (81%), US (94%), and Japan (95%).7-9,14 Gastrointestinal (GI) symptoms were seen in around 25.0% of our patients. Similarly, vomiting (25%) and diarrhea (25%) were the most common gastrointestinal manifestations seen in infected patients in the first wave of pandemic H1N1 in the US.15 GI symptoms in influenza infections can be attributed to direct viral effect on GI tract or systemic inflammatory response.16
In general, complications related to influenza infection are seen in the extreme age groups (adults ≥ 65 and children < 2 years old), pregnant women (up to two weeks postpartum), residents of long-term care facilities, and in people with certain medical conditions [Table 3].17
Table 3: Medical conditions that put people at high risk of developing influenza-related complications.
|
Chronic lung disease (e.g. asthma, chronic obstructive pulmonary disease) |
|
Heart disease (e.g. congenital heart diseases, congestive heart failure) |
|
Hematological diseases (e.g. sickle-cell disease) |
|
Chronic neurological disease (e.g. epilepsy) and neurodevelopmental conditions (e.g. mental retardation) |
|
Diabetes mellitus |
|
Chronic kidney disease |
|
Chronic liver disease |
|
Immunosuppression (due to disease or treatment) |
|
Morbid obesity (body Mass Index ≥ 40 kg/m2) |
|
Metabolic disorders (e.g. inherited metabolic disorders or mitochondrial disorders) |
|
People < 19 years old on long-term aspirin therapy |
Among the 51% of patients who had comorbidities, chronic lung disease or hematological disease was the main comorbidity putting the patient at risk of severe infection. This highlights the importance of starting empirical treatment with oseltamivir in such patients when presenting with pneumonia especially during the influenza season.
The abnormal laboratory findings that we have reported are consistent with the findings from previous studies among hospitalized patients: lymphopenia ranged between 20% and 68% anemia, and thrombocytopenia was noted in 37% and 14% of patients, respectively.9,11,12 Patients with severe disease had profound abnormal laboratory findings compared to mild cases, although it was not statistically significant [Figure 2]. For example, lymphopenia and thrombocytopenia were noted in 50% and 23%, respectively, in the severe disease compared to 36% and 14%, respectively, in the mild cases. Elevated ALT was also seen more in severe cases compared to mild cases (40% vs. 28%). This supports what has been observed in a larger study on 511 hospitalized patients in India where patients with severe H1N1 disease were more likely to have lymphopenia, thrombocytopenia, and transaminitis compared to hospitalized patients with the non-severe disease.13 These laboratory markers might aid in identifying patients who can be at a higher risk of developing complications.
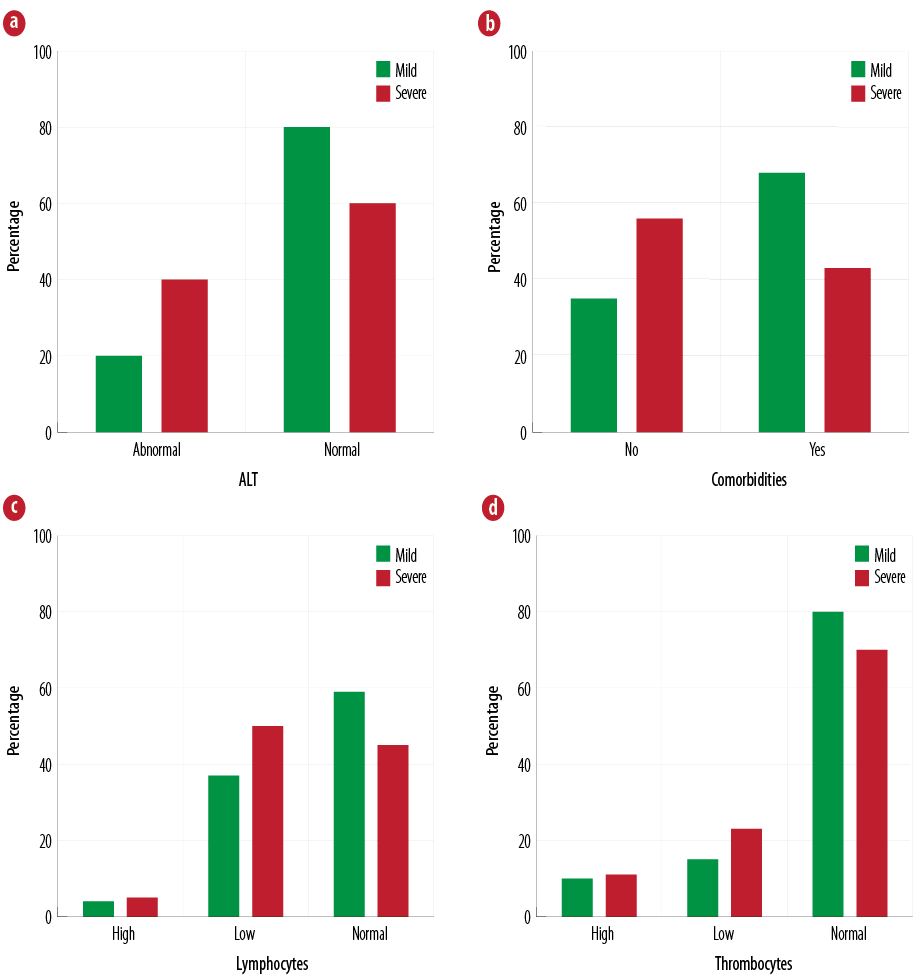
Figure 2: Classification of (a) ALT, (b) comorbidities, (c) lymphocytes, and (d) thrombocytes according to the category of severity.
Among the 56 patients who had a chest X-ray on admission, 50.0% had abnormal findings including lobar or bilateral consolidations. Other health institutions reported similar radiological findings in Oman (58–65%).7–9 A larger study conducted on 833 hospitalized infected patients in California in 2009, demonstrated that 66% had infiltrates suggestive of pneumonia or acute respiratory stress syndrome (ARDS).18 These data show that the majority of infected admitted patients have abnormal chest X-ray findings compatible with pneumonia as a result of a direct viral effect or secondary bacterial infection.
The US Centers for Disease Control and Prevention (CDC) recommends empirical treatment for people with suspected or confirmed influenza and illness requiring hospitalization, progressive/severe or complicated illness regardless of previous health status, and/or patients at risk of severe disease. All of our patients were treated with oral oseltamivir. One patient had both oseltamivir and nebulized zanamivir. This patient had severe ARDS and multi-organ failure and eventually died. The median time of treatment initiation was 2.5 days after disease onset. However, our patients were still offered treatment even when patients presented up to 14 days after the onset of illness. The administration of oseltamivir more than 48 hours after the onset of illness was associated with reduced mortality among 2009 H1N1 virus infected hospitalized patients.19 In addition, a meta-analysis of 29,234 patients found that administration of neuraminidase treatment, irrespective of timing, was associated with a reduction in mortality risk when compared to a group of patients who had no treatment.20 This might help reduce the burden of the virus in the respiratory tract and therefore the outcome. The rapid introduction of the influenza A H1N1 PCR test into SQUH enabled the quick identification and management of infected patients. However, antiviral treatment should not be delayed while awaiting laboratory confirmation for patients presenting with acute respiratory illness who are at risk of developing complications.
Corticosteroids have been used in the management of severe influenza A H1N1 infection. It is given mainly to control the inflammatory process and cytokine dysregulation causing lung injury and multi-organ dysfunction.21 However, there are mixed results about its effectiveness, and therefore, its role is unclear due to lack of randomized controlled trials. However, a meta-analysis review, looking at the efficacy of corticosteroids for the prevention of mortality in H1N1 infection, concluded that use of corticosteroids has negative or no effects on H1N1 treatment. Its use has been associated with increased mortality, increased risk of developing critical illness and secondary bacterial infection, and ICU admission or more prolonged ICU stays.22 Among our cohort, 11 patients received steroids and five were in the ICU. Here we have shown that the use of corticosteroids was significantly associated with a longer hospital stay (95% CI 2.06). The effect on mortality cannot be concluded due to the small size of our cohort and one death. Moreover, our multivariable analysis demonstrated that the severity of illness, presence of anemia (95% CI 1.60), lymphopenia (95% CI 1.72), and abnormal ALT (95% CI 1.83) increased the length of hospital stay.
The limitations of our study were mainly due to the small sample size included and its retrospective nature resulting in missing some clinical and laboratory details of some patients. However, the findings are in line with other published data worldwide. Also, we demonstrated that, in all cases, the length of hospital stay in patients taking steroids was significantly increased
Conclusion
The majority of our hospitalized patients were young and had comorbidities leading to complicated influenza infection requiring hospitalization. This highlights the importance of annual seasonal influenza vaccination to patients at high risk of influenza-related complications. However, this will always be challenged by the possible emergence of a novel influenza virus. The role of steroids in the management of severe influenza infection (including influenza A H1N1) is controversial. Further controlled studies are needed to make a recommendation on the use of such adjunctive therapy in the management of influenza infection.
Disclosure
The authors declared no conflicts of interest. No funding was received for this work.
references
- 1. CDC. Outbreak of Swine-Origin Influenza A (H1N1) Virus Infection --- Mexico, March--April 2009 [Internet]. cdc.gov. 2009 [cited 2015 Jul 9]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0430a2.htm.
- 2. WHO. Swine influenza: Statement by WHO Director-General, Dr Margaret Chan [Internet]. who.int. 2009 [cited 2015 Jul 9]. Available from: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090425/en/.
- 3. WHO. World now at the start of 2009 influenza pandemic: Statement to the press by WHO Director-General Dr Margaret Chan [Internet]. who.int. 2009 [cited 2015 July 9]. Available from: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/.
- 4. Pandemic WH. (H1N1) 2009 - update 88 [Internet]. who.int. 2010 [cited 2015 July 10]. Available from: http://www.who.int/csr/don/2010_02_19/en/.
- 5. Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Elsevier Health Sciences; 2014.
- 6. Al-Mahrezi A, Samir N, Al-Zakwani I, Al-Muharmi Z, Balkhair A, Al-Shafaee M. Clinical characteristics of influenza A H1N1 versus other influenza-like illnesses amongst outpatients attending a university health center in Oman. Int J Infect Dis 2012 Jul;16(7):e504-e507.
- 7. Pajankar S, Al Qassabi SS, Al Harthi SM. Clinical Features and Outcome of 65 Laboratory Confirmed Cases of H1N1 in Muscat, Sultanate of Oman. Oman Med J 2012 May;27(3):201-206.
- 8. Ahmad AS, Puttaswamy C, Mudasser S, Abdelaziz O. Clinical Presentation and Outcome in Hospitalized Patients of 2009 Pandemic Influenza A (H1N1) viral infection in Oman. Oman Med J 2011 Sep;26(5):329-336.
- 9. Al-Lawati J, Al-Tamtami N, Al-Qasmi A, Al-Jardani A, Al-Abri S, Busaidy Al S. Hospitalised patients with Influenza A (H1N1) in the Royal Hospital, Oman: Experience of a tertiary care hospital, July–December 2009. Sultan Qaboos University medical journal. Sultan Qaboos University 2010 Nov;10(3):326-334.
- 10. Clinical Management Of Human Infection With Pandemic. Who.int. N.p., 2009. [cited 2015 July 9]. Available from: http://www.who.int/csr/resources/publications/swineflu/clinical_management/en/.
- 11. Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al; 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 2009 Nov;361(20):1935-1944.
- 12. Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, et al; National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group of China. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med 2009 Dec;361(26):2507-2517.
- 13. Chudasama RK, Patel UV, Verma PB. Characteristics of Hospitalized Patients with Severe and Non-Severe Pandemic Influenza A (H1N1) in Saurashtra Region, India (Two Waves Analysis). J Family Med Prim Care 2013 Apr;2(2):182-187.
- 14. Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, et al; Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010 May;362(18):1708-1719.
- 15. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al; Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 Jun;360(25):2605-2615.
- 16. Minodier L, Charrel RN, Ceccaldi P-E, van der Werf S, Blanchon T, Hanslik T, et al. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol J 2015;12:215.
- 17. Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Jan;60(1):1-24.
- 18. Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al; California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009 Nov;302(17):1896-1902.
- 19. Yang SG, Cao B, Liang LR, Li XL, Xiao YH, Cao ZX, et al; National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group of China. Antiviral therapy and outcomes of patients with pneumonia caused by influenza A pandemic (H1N1) virus. PLoS One 2012;7(1):e29652.
- 20. Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TS, Al Mamun A, et al; PRIDE Consortium Investigators. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014 May;2(5):395-404.
- 21. Hui DS, Lee N, Chan PK. Adjunctive therapies and immunomodulatory agents in the management of severe influenza. Antiviral Res 2013 Jun;98(3):410-416.
- 22. Zhang Y, Sun W, Svendsen ER, Tang S, MacIntyre RC, Yang P, et al. Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit Care 2015;19:46.