Otomycosis, also known as fungal otitis externa, are fungal infections of the external auditory canal and seldom involve the middle ear. Despite being a benign condition, eradication of this entity remains a challenge to medical practitioners especially otorhinolaryngologists. Aspergillus spp. and Candida spp. are the most frequent isolated fungi in otomycosis.1 To date, there has been no standardized treatment regime for otomycosis, which opens up new treatment options including the use of
herbal medicine.
Throughout the globe, many plants have been utilized for their medicinal properties. Aloe vera species has been used in folk medicine for over 2000 years and has remained an important component in the traditional medicine of many countries. Aloe barbadensis miller also known as Aloe vera is one of more than 400 species of Aloe vera and belongs to the Liliaceae family.2 Aloe vera’s prominent feature is its high water content, which ranges from 99.0−99.5%. The remaining 0.5−1.0% is reported to contain over 75 nutrients and 200 active compounds including sugar, anthraquinones, saponins, vitamins, enzymes, minerals, lignin, salicylic acid and amino acids, and other different potentially active compounds including water-soluble and fat-soluble vitamins, minerals, enzymes, simple/complex polysaccharides, phenolic compounds, and organic acid.3 Aloe vera has two parts, the outer rind and the inner colorless parenchyma aloe gel. Both parts of Aloe vera have medicinal values. Based on in vitro and animal studies, which used total leaf extract, Aloe vera exhibits anti-inflammatory, anti-arthritic, antibacterial, and hypoglycemic properties.4 Several studies have proven the antifungal properties of Aloe vera extract.5 This pilot study aimed to determine the antifungal properties of Malaysian Aloe vera leaf extract on otomycosis species including Aspergillus niger and Candida albicans.
Methods

Figure 1: (a) Aloe vera leaves dried at 45 ºC in a hot air oven. (b) Soxhlet apparatus used for extraction.
(c) Candida albicans and Aspergillus niger culture.
This laboratory-controlled prospective study was conducted in the microbiology and pharmacology laboratory of the Universiti Sains Malaysia and was approved by the institution’s Ethics Committee.
Aloe vera leaves collected from a single area were washed with distilled water before being oven dried at 45 ºC for three to five days [Figure 1a]. Dried Aloe vera leaves were ground to powder form and stored in a tightly sealed container. The Soxhlet apparatus and method was used for extraction [Figure 1b].6 Two forms of solvents were used: aqueous and 70% ethanol. The Soxhlet thimble was filled with the powdered leaves and inserted into the Soxhlet main chamber and closed. One liter of 70% ethanol was filled into the Soxhlet main chamber and attached to the Soxhlet apparatus, which was heated until the solvent vapor filled the main chamber. The solvent vapor then condensed and dripped back down into the chamber containing the Aloe vera leaf extract. The Aloe vera leaf extract using 70% ethanol was then evaporated with a rotary evaporator at 30 oC and concentrated to 50 mL before being freeze-dried. The powdered form of freeze-dried extract was kept in the freezer to maintain the compound. For the aqueous extract, the same extraction technique was used using 70% distilled water as a solvent instead of ethanol. The powdered form of Aloe vera leaf extracts were then used to establish five different concentrations by serial dilution (50 g/mL, 25 g/mL, 12.5 g/mL, 6.25 g/mL, and 3.125 g/mL) using a starting concentration of 50 g/mL.
The tested fungal isolates used in this study were C. albicans and A. niger from otomycosis and were obtained from the archives of Microbiology Laboratory of the School of Medical Sciences, Universiti Sains Malaysia. A. niger and C. albicans grown on Sabouraud dextrose agar (SDA) plates [Figure 1c] were suspended in sterile distilled water and adjusted to 1 × 106 colony forming units (CFU/mL) (0.5 McFarland standard) using a nephelometer.
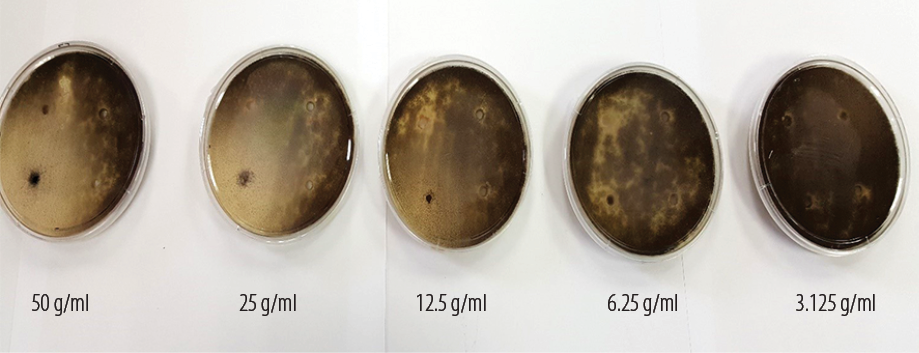
Figure 2: Zones of inhibition of Aspergillus niger for five different aqueous extract Aloe vera leaf concentrations.
The standardized fungal isolates were used to lawn the SDA plates using sterile swabs after diluting the organisms for 15 minutes. The SDA plates lawned with fungal isolates were then divided into four equal quadrants. With the help of a sterile glass pipette, four equal wells were created. The different concentrations of Aloe vera leaf extract (100 µL) were transferred into the well using a micropipette. The aqueous Aloe vera extract was transferred to the upper quadrant of the well with its control aqueous solution at the opposite site, and the alcohol extract of the Aloe vera was transferred to the lower quadrant with its control in the opposite site. The SDA plates were then kept lid side up in a 30 ºC incubator. The plates were replicated five times.
The plates were checked daily for spillage and growth of other organisms. Measurement of the inhibition zone was done on the third day when the margin of inhibition was clearly visible [Figure 2]. The zones of complete inhibition was measured using a Vernier caliper in millimeters by gross visual inspection. To determine the minimum inhibitory concentration (MIC) of each extract, the agar diffusion method was used.6 After measuring the zone of inhibition, a scattered plot graph X2 versus log concentration was used to determine the MIC level of the Aloe vera extract.
Results
We sought to evaluate the antifungal properties of different concentrations of Aloe vera leaf extracts on A. niger and C. albicans cultures. Both the zone of inhibition of A. niger and C. albicans in alcohol and aqueous extracts of Malaysian Aloe vera leaf were determined and the MIC for each extract
was calculated.
The zone of inhibition (i.e., the zone with no fungal organism growth) was measured for each of the tested alcohol and aqueous Aloe vera leaf extracts. Five different concentrations of alcohol and aqueous Aloe vera leaf extracts were tested for five replicates. The results obtained were within a close range for the five replicates for each concentration.
For A. niger cultures, the zone of inhibition was noted after three days incubation for all concentrations except 3.125 g/mL for both alcohol and aqueous extracts, which failed to show any zone of inhibition [Table 1]. The highest inhibition zone was seen with the 50 g/mL concentration for both alcohol and aqueous extracts. A. niger showed significant zone of inhibition for both alcohol and aqueous extracts of Aloe vera for 50 g/mL, 25 g/mL, 12.5 g/mL and 6.25 g/mL. However, there was no zone of inhibition seen with for 3.125 g/mL concentration for both aqueous and alcohol extracts. There was an increase in the zone of inhibition proportional to the increase in the extract concentration. One-way analysis of variance (ANOVA) was used to establish the mean difference between groups [Table 2]. There was a significant difference between the four groups (p < 0.001).
Table 1: Zone of inhibition of different concentrations of aqueous and alcohol Aloe vera leaf extracts.
|
Aqueous |
50.0 |
14 |
18 |
16 |
16 |
16 |
16 |
| |
25.0 |
14 |
14 |
16 |
12 |
14 |
14 |
| |
12.5 |
8 |
6 |
8 |
8 |
8 |
7.6 |
| |
6.25 |
8 |
8 |
6 |
6 |
6 |
6.8 |
| |
3.125 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Alcohol |
50.0 |
35 |
35 |
34 |
35 |
35 |
35 |
| |
25.0 |
35 |
35 |
34 |
33 |
33 |
33.8 |
| |
12.50 |
20 |
22 |
23 |
20 |
20 |
21 |
| |
6.25 |
10 |
8 |
10 |
10 |
10 |
9.6 |
Table 2: Mean zone of inhibition against Aspergillus niger for aqueous and alcohol extracts of Aloe vera.
|
50.0 |
16.0±1.4 |
168.467 (4) |
< 0.001 |
34.8±0.4 |
1443.950 (4) |
< 0.001 |
|
25.0 |
14.0±1.4 |
|
|
34.0±1.0 |
|
|
|
12.5 |
7.60±0.9 |
|
|
21.0±1.4 |
|
|
aScheffe test.
Scheffe test post hoc comparison was done to define the pairs of concentrations with a significant difference. Higher mean zones of inhibition were observed in 50.0 g/mL and 6.25 g/mL followed by 50.0 g/mL and 12.5 g/mL, 25.0 g/mL and 6.25 g/mL and, finally, 25.0 g/mL and 12.5 g/mL. The mean zone of inhibition increased in proportion to the concentration. For the alcohol extracts, the mean differences between groups of concentration showed that at higher concentrations there was a greater effect on the mean zone of inhibition [Table 2].
Post hoc comparison results revealed that higher mean zones of inhibition were observed in 50.0g/mL and 6.25 g/mL followed by 25.0 g/mL and 12.5 g/mL, 50.0 g/mL and 12.5 g/mL, 25.0 g/mL and 12.5 g/mL, and 12.5 g/mL and 6.25 g/mL. The higher the concentration of Aloe vera alcohol extract the greater the mean zone of inhibition. There was a significant mean difference between both the aqueous and alcohol extracts.
The independent t-test was used to compare the means of the two groups of extracts. Alcohol extracts had a higher mean zone of inhibition compared to aqueous extracts (p < 0.001) against A. niger and 95% confidence intervals of mean difference did not include zero. Hence, it can be concluded that the antifungal effect of A. niger is statistically better in alcohol than aqueous extracts [Table 3]. For C. albicans, there was no zone of inhibition seen for both alcohol and aqueous extracts.
Table 3: Comparison of mean of zone inhibition between aqueous and ethanol extracts of Aloe vera against the growth of Aspergillus niger at five different concentrations.
|
50.0 g/mL |
Aqueous |
16.0±1.4 |
-18.8 (-20.3,-17.3) |
-28.342 (8) |
< 0.001 |
| |
Alcohol |
34.8±0.4 |
|
|
|
|
25.0 g/mL |
Aqueous |
14.0±1.4 |
-20.0 (-21.8,-18.2) |
-25.820 (8) |
< 0.001 |
| |
Alcohol |
34.0±1.0 |
|
|
|
|
12.5 g/mL |
Aqueous |
7.6±0.9 |
-13.4 (-15.1,-11.7) |
-17.907 (8) |
< 0.001 |
| |
Alcohol |
21.0±1.4 |
|
|
|
|
6.25 g/mL |
Aqueous |
6.8±1.1 |
-2.80 (-4.3,-1.3) |
-4.000 (8) |
< 0.001 |
| |
Alcohol |
9.6±0.9 |
|
|
|
*t-statistics could not be determined as standard deviations for both groups were 0; aIndependent t-test; CI: confidence interval; n/a: non-applicable. No zone of inhibition seen.
Calculation of the MIC was carried out using agar diffusion method with a scattered plot graph of X2 versus log concentration. The MIC of the aqueous extract was 5.1 g/mL and alcohol extract was
4.4 g/mL [Figure 3].
Discussion
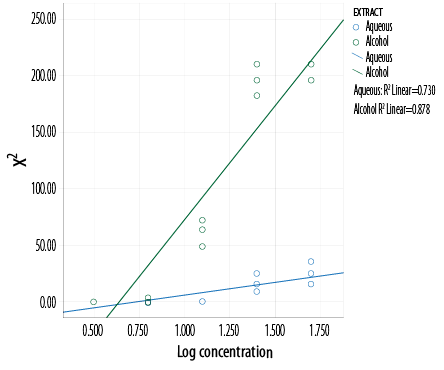
Figure 3: Determination of the MIC using a scattered plot graph of X2 versus log concentration.
We sought to evaluate the in vitro antifungal effect of Malaysian Aloe vera leaf extracts in alcohol and aqueous solutions on two common pathogenic otomycosis species, A. niger and C. albicans, using the zone of inhibition and MIC to determine antimicrobial activity. We found that both alcohol and aqueous extracts demonstrated notable antifungal properties against A. niger.
The antifungal effect of this study was solvent dependent. The highest concentrations of alcohol and aqueous extracts displayed the maximum zone of inhibition. C. albicans showed resistance to both the alcohol and aqueous Aloe vera extracts at different concentrations. The antifungal effect of Aloe vera leaf may vary according to solvent. In our study, the antifungal property of the alcohol extract was more potent than the aqueous extract. The MIC of the alcohol extract was 4.4 g/mL compared to the aqueous extract which was 5.1 g/mL. Similarly, a study to evaluate antimicrobial and antifungal activity of Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, A. niger, and C. albicans, found that alcohol extracts at higher concentrations were more potent than petroleum ether and chloroform extracts.7 A study by Devi et al,8 on the antimicrobial activity of dimethyl sulfoxide (DMSO) crude extracts of Aloe barbadensis miller gel against selected bacterial and fungal pathogens including A. niger and C. albicans was carried out using the disc diffusion method at three different concentration (100, 200, and 400 µg/mL). The extracts failed to show a zone of inhibition at any concentration to A. niger compared to C. albicans, which showed a significant zone of inhibition in proportion to concentration. Another study concluded that ethanol extracts had a better MIC compared to aqueous and methanol extracts for E. coli, S. aureus, and C. albicans.9 The authors also noted that variations in antimicrobial activity depended on the extraction method used. The authors used scrapped Aloe vera gel, which was ground and mixed with 100 mL of each solvent (ethanol, aqueous, and methanol) separately and left for 72 hours after being filtered to obtain the extracts for the study.
We found no zone of inhibition of C. albicans for both the alcohol and aqueous extracts for all five concentrations tested. This was also observed in a study by Khaing et al.10 The authors used the agar diffusion method and crude Aloe vera leaf extracts in methanol, ethanol, and ethyl acetate and found that all three extracts had no zone of inhibition for the C. albicans. As for A. niger, the zone of inhibition was highest for the methanol extract followed by ethanol and ethyl acetate extracts. According to a study by Shekrawat et al,11 alcohol extract was effective secondary to its constituent of extraction.
The resistance of C. albicans towards Aloe vera leaf extracts in our study compared to other regions may be due to geographical and climatic conditions, which may affect the phytochemical composition of the plant and its antifungal activity. A study by Kumar et al,5 showed a role of climate and geography on variations in the amount of aloe-emodin (anthraquinones compound found in Aloe vera) hence the variable antimicrobial effect.5 Aloe vera leaves from six different climatic regions of India were extracted using methanol and tested against nine bacterial and two fungal strains (C. albicans and A. niger). Overall, methanolic extracts from six different climatic regions exhibited good antimicrobial and antifungal activity; however, variations in the zone of inhibition were due to difference in the phytochemical composition of the plant from different climatic conditions.
Antifungal activity of ethanolic extracts at six different concentrations (400, 200, 150, 100, 50, and 25 g/mL) of three local plants of Iran (Elettaria cardamomum, Aloe vera, and Thymus vulgaris) against C. albicans revealed that Elettaria cardamomum and Aloe vera had significant inhibitory properties. The Thymus vulgaris extract showed no activity.12 Although we used alcohol extract in our study, this was prepared by the Soxhlet extraction method rather than the grinding and filtering method used by Al-Hussaini, et al.12 Preparation of the Aloe vera extract at high temperature may have affected the active ingredient leading to the ineffectiveness towards C. albicans.13 Additionally, a study by Qasem et al,14 on the fungicidal activity of some weed extracts against different plant pathogenic fungi revealed that the technique of extraction, solvent type, and age of plant might predispose a difference in its active composition and antifungal activity.
Aloe vera has been proven to contain mono- and polysaccharides, tannins, sterols, organic acids, enzymes, saponins, vitamins, and minerals. Arunkumar and Muthuselvam15 published an analysis of the phytochemical constituents and antimicrobial activities of Aloe vera leaf using aqueous, ethanol, and acetone extracts against selected human pathogens. The authors identified 26 bioactive phytochemical compounds. Plant compounds including anthraquinones, dihydroxyanthraquinones, saponins, acemannan, and aloe-emodin have been proposed by various studies to have both direct and indirect antimicrobial properties.16
Conclusion
Our study may aid to establish which naturally sourced compounds can be used to formulate new and more potent antifungal agents against otomycosis. The problem of increasing microbial resistance has made it prudent to identify natural antimicrobial compounds. The phytochemical composition of the Malaysian Aloe vera plant should be studied further using different extraction methods, which may result in better antifungal effects.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Vennewald I, Klemm E. Otomycosis: Diagnosis and treatment. Clin Dermatol 2010 Mar;28(2):202-211.
- 2. Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol 1999 Dec;68(1-3):3-37.
- 3. Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med 2014 Dec;5(1):21-26.
- 4. Newall CA, Anderson LA, Philipson JD. Herbal medicines: A guide for health-care professionals. London: The Pharmaceutical Press. 1996;25.
- 5. Kumar S, Yadav M, Yadav M, Yadav JP. Comparative analysis of antimicrobial activity of methanolic extracts of Aloe vera and quantification of Aloe Emodin collected from different climatic zones of India. A C Microb. 2015;(6)2:1.
- 6. Arumugam PA, Mohamad I, Salim R, Mohamed Z. Antifungal effect of Malaysian Neem Leaf extract on selected fungal species of pathogenic otomycosis in vitro culture medium. Malays J Med Health Sci. 2015;11(2):69-84.
- 7. Kedarnath, Kaveri KM, Chimkod VB, Patil CS. Antimicrobial activity of Aloe vera leaf extract. Int J Biol Pharm Tech. 2013;4(4):286-290.
- 8. Devi DL, Srinivas B, Rao BN. An evaluation Antimicrobial Activity of Aloe barbadensis Miller (Aloe vera) Gel Extract. J Pharm Biomed Sci 2012;21(3):1-4.
- 9. Mbajiuka CS, Obeagu EI, Okwandu GE. Antimicrobial effext of Aloe vera on some human pathogens. Int J Curr Microbio App Sci. 2014;3(3):1022-1028.
- 10. Khaing TA. Evaluation of Antifungal and antioxidant activities of leaf extract of Aloe vera. World Acad Sci Eng Technol 2011;5:610-612.
- 11. Shekrawat PS, Prasada R. Antifungal properties of some plant extracts: Inhibition of spore germination. Indian Phytopathol. 1971;24:800-802.
- 12. Al-Hussaini JS, Al-Mohana AM. An evaluation of the antifungal activity of some local medicinal plants against growth of Candida albicans in vitro. Al-Qadisiya J Vet Med Sci. 2010;9(2):60-68.
- 13. Kaur H, Goyal RR, Bhattacharya A, Gupta R, Lal NK, Arora B, et al. Antifungal activity of phytoextracts of Piper longum, Aloe vera, and Withania somnifera against human fungal opportunistic pathogen Candida albicans. DU Journal of Undergraduate Research and Innovation. 2015;1-9.
- 14. Qasem J, Aau-Blan HA. Fungicidal activity of some common weed extracts against different plant pathogenic fungi. J Phytopathol 1996;144(3):157-161.
- 15. Arunkumar S, Muthuselvam M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci 2009;5(5):572-576.
- 16. Cock IE. Antimicrobial Activity of Aloe barbadensis Miller Leaf Gel Components. Int J Microbiol 2008;4:2.