Extraskeletal myxoid chondrosarcoma (ESMC) is a soft tissue malignancy with a predilection for soft tissues of long bones.1,2 ESMC is exceedingly rare in the nasopharynx, posing a diagnostic and therapeutic dilemma due to its location, vast array of differential diagnosis, and only one previously reported case3 without any definite therapeutic guidelines. Wide excision of the lesion is regarded as the initial treatment of choice.1 Radiotherapy remains the cornerstone of adjuvant therapy due to the high probability of local recurrence1,2 and also as a palliative option in metastatic or progressive cases.4
Case report
A 60-year-old female presented with left-sided nasal obstruction and occasional epistaxis of one-year duration. Nasal endoscopy showed a pink, smooth, polypoid, fleshy growth occupying the whole of the nasopharynx. Biopsy suggested a low-grade tumor favoring myxoid chondrosarcoma. Magnetic resonance imaging [Figure 1] showed a 3.5 × 3.4 × 6.7 cm well-defined hyperintense expansile soft tissue lesion arising from the nasopharynx, invading sphenoid and clivus. Cervical lymphadenopathy was absent. Metastatic workup was negative.
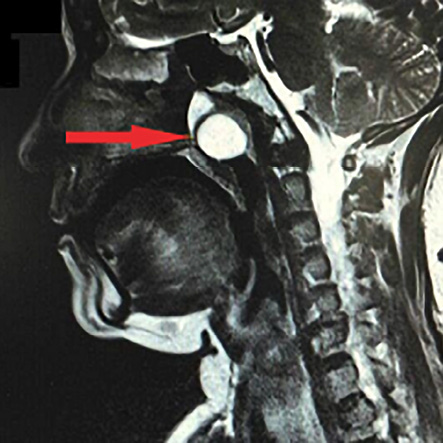
Figure 1: Sagittal section of T2-weighted magnetic resonance imaging showing well-defined hyperintense expansile soft tissue lesion arising from the nasopharynx, invading sphenoid and clivus (red arrow).
The patient underwent endoscopic resection with piecemeal excision of the tumor. Histopathological report (HPR) revealed tumor cells arranged in cords, reticulae, strands, and trabeculae interspersed within a myxoid stroma. The tumor cells were spindle- to stellate-shaped with round to oval hyperchromatic nuclei and scanty eosinophilic cytoplasm. Resection margins were positive for tumor. Immunohistochemistry was positive for vimentin and synaptophysin [Figure 2] while negative for pan-Cytokeratin (panCK), P-40, S-100, epithelial membrane antigen (EMA), chromogranin, melan-A, desmin, smooth muscle actin (SMA), neuron-specific enolase, CD99, CD56, CD20, CD45, and glial fibrillary acid protein (GFAP), thus confirming ESMC. Given the gross residual disease and positive resection margins, the patient was treated with adjuvant conformal radiotherapy to a dose of 66 Gray (Gy) in 33 fractions to which she showed excellent symptomatic and radiological improvement [Figure 3]. Presently she is on follow-up for over two years without any evidence of local recurrence or distant metastasis.

Figure 2: Hematoxylin and eosin staining revealed (a) Histopathological report showing tumor cells arranged in cords, reticular pattern, strands, and trabeculae interspersed in a myxoid stroma, magnification = 40 ×. (b) Positive staining for vimentin, magnification = 20 × and (c) synaptophysin, magnification = 10 ×.
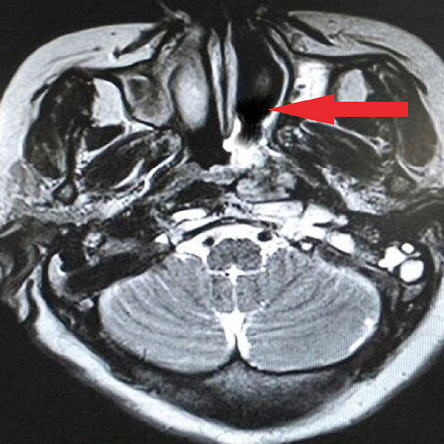
Figure 3: Axial section of magnetic resonance imaging post-adjuvant radiotherapy showing a significant radiological response.
Discussion
Chondrosarcoma is a slow-growing malignant tumor of skeletal and extraskeletal origin comprising less than 1% of all head and neck tumors.4 Chondrosarcoma has several histological variants, with the myxoid subtype having a predominant soft tissue origin accounting for less than 10% cases5 and less than 5% of all head and neck malignancies.5 ESMCs most commonly involve the soft tissue of long bones of lower extremities.1,2 Other sites affected include the lung, spine, scrotum, testis, synovium, mammary gland, vulva, and abdominal wall.1
There have been few case reports of ESMC of the oropharynx, masticator space, infratemporal fossa, cerebellopontine angle, orbit, chin, nasal septum, nasal cavity, buccal space, and sphenoid sinus. Nimonkar et al,6 treated a solitary case of ESMC of the maxilla in a 12-year-old girl with surgery and adjuvant radiotherapy with disease-free survival (DFS) of 10 months. Jérôme-Marson et al,7 reported two cases of ESMC involving nasal cavity and sphenoid sinus. In both cases curative surgery was not possible and adjuvant radiotherapy was given. Ganguly and Mukherjee1 reported a case of maxillofacial ESMC managed with surgery and radiotherapy with DFS of one year while Zaki et al,8 reported one case of ESMC of neck treated with chemoradiation. Following an extensive literature search, we could find only one case of nasopharyngeal ESMC reported in 2011 by Bhalla and Osipov.3 Here, we present the second case of ESMC of nasopharynx reported to date.
The exact etiology of ESMC is still unknown.1,2 Several theories like primitive cartilage forming mesenchyme, chondroblastic differentiation, synovial intimal cells, past surgical or traumatic insult, inhalation of chemical carcinogens,1 multiple hereditary exostoses, intravenous thorotrast, previous irradiation, Maffucci syndrome, and Ollier disease4 have been postulated, but none has been considered satisfactory. No association has been found with Epstein-Barr virus or consumption of salted fish.
ESMC is known to metastasize to lungs, bones, brain, lymph nodes, and testes.1 Primary nasopharyngeal ESMC can often be misdiagnosed with squamous cell carcinoma nasopharynx, melanomas, lymphomas, soft tissue sarcomas, Ewing sarcoma, skeletal chondrosarcomas, chordomas, parachordomas, myoepithelial carcinoma, myxopapillary ependymomas, chordoid meningiomas, and myxoid liposarcoma.1
Microscopic features of cords and lobules of neoplastic cells dispersed within a myxoid matrix are characteristic of ESMC.9 Individual tumor cells are often round to oval with scant or moderate eosinophillic cytoplasm and pleomorphic nuclei with rhabdoid and epitheloid cells scattered at periphery.9 ESMC expresses neuroendocrine differentiation with strong immunopositivity for vimentin and synaptophysin7and weak or focal positivity for EMA and S-100.7 Myxoid matrix with lobulated neoplastic cells is also seen in chordoma, parachordoma, chordoid meningiomas, and myxopapillary ependymoma. However, chordomas stain positive for panCK and EMA and negative for GFAP while parachordomas are positive for EMA, S-100, panCK, and vimentin with abundant type IV collagen.
Chordoid meningiomas are positive for EMA, negative for panCK and GFAP while myxopapillary ependymomas are positive for GFAP and negative for EMA. Myxoidstroma with vimentin positivity is seen in myxoid liposarcoma, but the presence of lipoblasts rules out ESMC. An eosinophillic cytoplasm is also present in myoepithelial carcinoma, but it shows positivity for panCK, SMA, and p63 apart from vimentin, S-100, and EMA. Demonstration of two translocations t(9;22) and t(9;17) by reverse transcription polymerase chain reaction is required to confirm the diagnosis of ESMC.1
Surgical excision has been the initial treatment of choice as chondrosarcoma is traditionally regarded a radio-resistant tumor.1 Since nasopharyngeal malignancies cannot be resected with wide surgical margins due to their anatomic location and proximity to critical structures, adjuvant radiotherapy plays a major role in treatment. Since no specific dosage schedule has been mentioned for nasopharyngeal ESMC, we used a dose of 66 Gy in 33 fractions given margin positivity. Chemotherapy has been effective in patients with metastatic or progressive ESMC2 and mesenchymal chondrosarcomas.4 Chemotherapeutic drugs like doxorubicin, ifosfamide, cisplatin, gemcitabine, dacarbazine, interferon-alpha, and imatinib have been utilized without any significant long-term effect.1 Sunitinib has been used in metastatic ESMC expressing EWSR1/TAF15-NR4A3 translocation with satisfactory results.10
Conclusion
The diagnosis of ESMC of nasopharynx should always be considered in patients presenting with nasal obstruction and epistaxis with a soft tissue mass on nasal endoscopy. A better interpretation of the molecular and biological mechanism of the disease process may help to devise therapeutic strategies to counter this entity. More sophisticated diagnostic techniques, improved cytogenetic analysis, novel targeted therapies, and optimal radiotherapy dosages should be devised to improve the disease-free and overall survival of patients.
Disclosure
The authors declared no conflicts of interest.
Acknowledgements
We thank the patient for allowing us to publish the case report and use the images taken during her stay in hospital. Written informed consent to publication was obtained from the patient. We also like to extend our gratitude to Departments of Otorhinolaryngology, Nuclear Medicine, and Radiology, Army Hospital (Research and Referral), New Delhi, India.
references
- 1. Ganguly R, Mukherjee A. Maxillo-facial Extraskeletal Myxoid Chondrosarcoma: A Case Report and Discussion. Korean J Pathol 2011;45:639-643.
- 2. Stacchiotti S, Dagrada GP, Sanfilippo R, Negri T, Vittimberga I, Ferrari S, et al. Anthracycline-based chemotherapy in extraskeletal myxoid chondrosarcoma: a retrospective study. Clin Sarcoma Res 2013 Dec;3(1):16.
- 3. Bhalla A, Osipov V. Extraskeletal myxoid chondrosarcoma of the nasopharynx. Pathology 2011 Aug;43(5):507-509.
- 4. Lee SY, Lim YC, Song MH, Seok JY, Lee WS, Choi EC. Chondrosarcoma of the head and neck. Yonsei Med J 2005 Apr;46(2):228-232.
- 5. Garde JB, Palaskar SJ, Kathuriya PT. Extraskeletal myxoid chondrosarcoma of maxilla: A rare entity. J Oral Maxillofac Pathol 2016 Jan-Apr;20(1):151-153.
- 6. Nimonkar P, Bhola N, Jadhav A, Jain A, Borle R, Ranka R, et al. Myxoid Chondrosarcoma of Maxilla in a Pediatric Patient: A Rare Case Report. Case Rep Oncol Med 2016;2016:5419737.
- 7. Jérôme-Marson V, Uro-Coste E, Lacoste-Collin L, Gomez-Brouchet A, Serrano E, Delisle MB. [Extraskeletal myxoid chondrosarcoma of the nasopharynx]. Ann Pathol 2003 Jun;23(3):253-257.
- 8. Zaki M, Laszewski P, Robinette N, Saleh H, Raza N, Sukari A, et al. Unresectable extraskeletal myxoid chondrosarcoma of the neck: Early tumor response to chemoradiotherapy. Cureus 2015 Dec;7(12):e432.
- 9. Oliveira AM, Sebo TJ, McGrory JE, Gaffey TA, Rock MG, Nascimento AG. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod Pathol 2000 Aug;13(8):900-908.
- 10. Stacchiotti S, Pantaleo MA, Astolfi A, Dagrada GP, Negri T, Dei Tos AP, et al. Activity of sunitinib in extraskeletal myxoid chondrosarcoma. Eur J Cancer 2014 Jun;50(9):1657-1664.