Cancer is the second cause of death in the world,1 and gastric cancer has a mortality of about 70% with a higher prevalence in men. In the US, the incidence of gastric cancer is 21000 cases annually, which lead to 10570 deaths.2 In Iran, the percentage of gastric cancer in 2005–2006 was 15.2% and 6.8% in men and women, respectively.3,4 Nanomaterials are small objects with at least one dimension in the nanoscale range (1–100 nm).5 They can have toxic effects on cancer cells and destroy them through oxidative stress.6,7 This feature can be utilized to develop effective methods to destroy tumors with minimal side effects. Nowadays, using nanoparticles in the diagnosis and treatment of cancer has been extensively studied.8,9 One feature of cancer cells is invasion of adjacent tissues.10 Epithelial-mesenchymal transition has a significant role in cancer cell invasion. Matrix metalloproteinases (MMPs) stimulate invasion and cancer cells can move to other tissues.11 Some studies have shown that nanoparticles can inhibit the invasion of cancer cells.12
Titanium dioxide (TiO2) nanoparticles have been shown to have antitumor effects, and inflammatory properties on mammalian cells through hydrogen peroxide (H2O2) and hydroxyl free-radical production.6,13–15 TiO2 causes apoptosis in human bronchial epithelial cells through launching reactive oxygen species (ROS).16–18 When human epithelial cell lines were exposed to TiO2 nanoparticles there was increased expression of Bax, cytochrome C and P53 proteins and a reduction of anti-apoptosis B-cell lymphoma (BCL2), which eventually led to apoptosis in these cells. In addition, the expression level of caspase-9 increased, but the expression of caspase-8 did not change. This suggests that TiO2 acts through an intrinsic pathway to cause apoptosis in human epithelial cells.16
We sought to determine the effect of TiO2 nanoparticles on apoptosis and invasion of gastric cancer cell line, MKN-45. We used the MTT assay and a wound healing assay to assess invasion and acridine orange and ethidium bromide (AO/EB) staining to test cell death. We used seven different TiO2 structures: Polyethylene glycol (PEG)-amorph TiO2, amorph TiO2, PEG-brookite, rutile, anatase, amorph bovine serum albumin (BSA), and brookite BSA. Anatase and rutile are two important TiO2 structures. Rutile is more stable and bigger than anatase.19 All nanoparticles were used at different concentrations (0, 10, 20, 30, 40, and 50 μg/mL), and the cells were exposed to the nanoparticles for 24, 48, and 72 hours).
Methods
Human gastric cancer cell line MKN-45 (NCBI No.C615) was purchased from Iran Pasteur Institute. The cells were cultured in RPMI 1640 (Gibco, Invitrogen GmbH, Darmstadt, Germany) included 10% fetal bovine serum (Gibco, Invitrogen GmbH, Darmstadt, Germany), 100 unit/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified incubator with 5% CO2. Amorph, brookite, anatase, and rutile forms of TiO2 nanoparticles (Grafen Chemical Industries, Ankara, Turkey) were coated with PEG and BSA.
We used the MTT assay to examine cell survival. MKN-45 cells were seeded in a flask; when the concentration reached to 60%, cells were trypsinized and moved to a 96 well plate. Each well contained 5000 cells in 200 µL RPMI 1640 medium. Cells were cultured for 24 hours after which they were exposed to the nanoparticles. Different concentrations of nanoparticles (0, 10, 20, 30, 40, and 50 μg/mL) were used for three different times (24, 48, and 72 hours). On the day of testing, 20 μL of MTT reagent was added to cells and the contents of each well were replaced with 100 μL dimethyl sulfoxide. Absorbance in each well at 570 nm was recorded using the Elisa reader system (Rayto software). Each experiment was triplicated. The results of the MTT assay were used to calculate the 50% inhibitory concentration (IC50).
MKN-45 cells were seeded in six-well plates and incubated with nanoparticles at the IC50 value for 48 hours. AO/EB reagent at 100 μg/mL was prepared, and cells were trypsinized and stained with 100 μL of AO/EB solution for 15 minutes and washed twice with phosphate buffered saline (PBS) solution and assessed by fluorescence microscopy. Green color indicated live cells, red represented apoptotic cell, and orange indicated necrotic cells.
A wound healing assay to examine the migration of MKN-45 cells was done. MKN-45 cells were seeded in six-well plates. After 24 hours, the center of well was scraped with a sterile micropipette tip to make a straight scratch with the same width. Each well was washed with PBS solution, and MKN-45 cells were exposed to TiO2 nanoparticles (at the IC50 value) and moved to an incubator for 48 hours. Wound closure was recorded with an inverted microscope.
Each assay was triplicated, and data expressed as the mean. Statistical analyses were performed using SPSS Statistics (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.). Graphs were prepared using Microsoft Excel 2007. After normality and homogeneity evaluation of variables, analysis of variance (ANOVA) was done with a 95% confidence interval (p ≤ 0.050); Tukey's test was used for multiple comparisons.
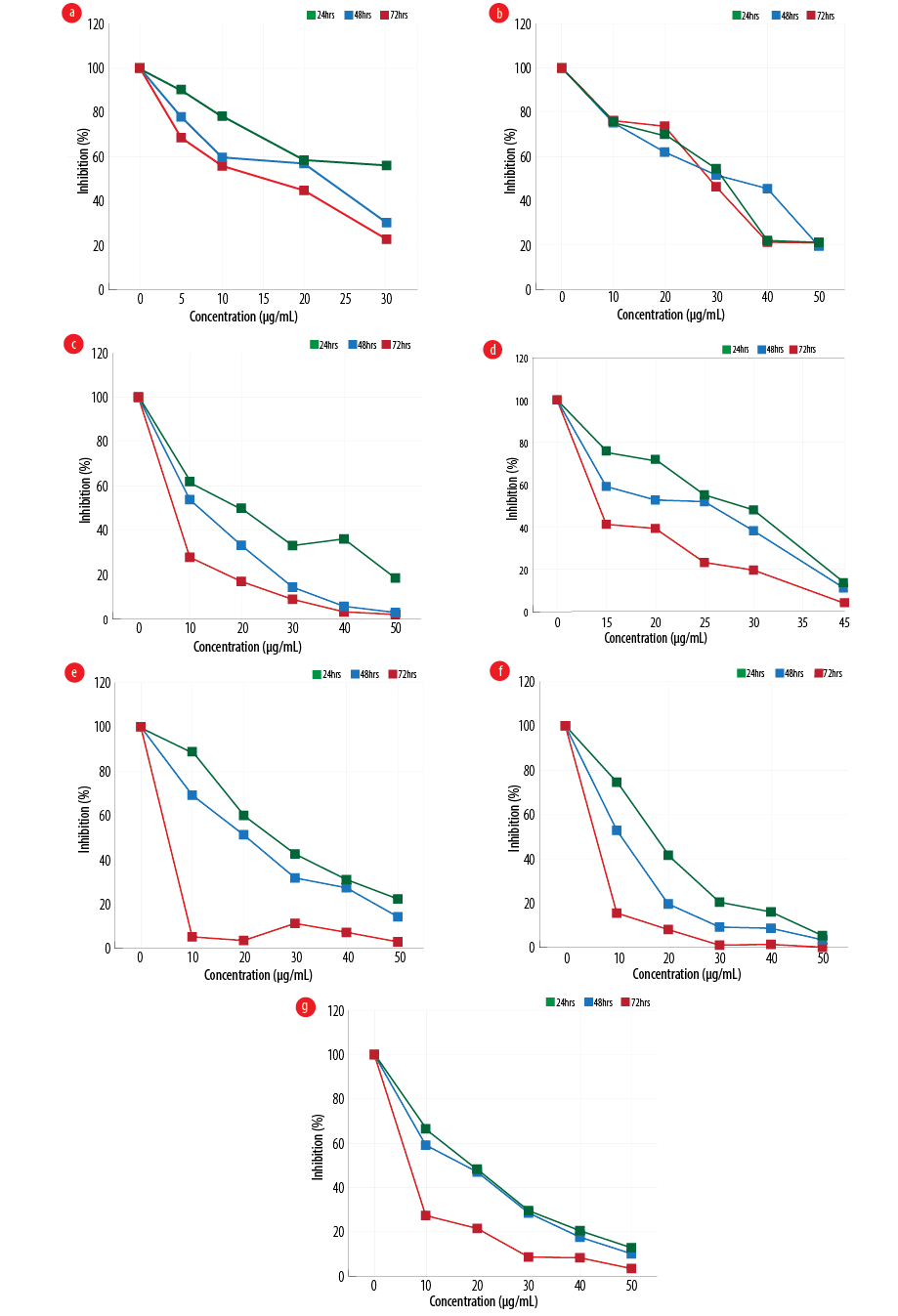
Figure 1: Effect of different configurations and coating of nanoparticles on the proliferation of gastric cancer line, MKN-45 (a) amorph titanium dioxide (TiO2), (b) polyethylene glycol (PEG)-amorph TiO2, (c) anatase, (d) PEG-brookite, (e) rutile, (f) brookite bovine serum albumin (BSA), and (g) amorph BSA.

Figure 2: Induction of apoptosis in cancer cells after exposure to different configurations and coatings of nanoparticles for 48 hours. In all groups, the concentration used was the 50% inhibitory concentration (IC50) value. Apoptosis, left (control), and right (IC50 dose at 48 hours). Green color indicated live cells, red apoptotic cells and orange necrotic cells. (a) polyethylene glycol (PEG)-brookite, (b) amorph titanium oxide (TiO2), (c) PEG-amorph TiO2, (d) rutile, (e) anatase, (f) brookite bovine serum albumin (BSA), and (g) amorph BSA.
Results
MKN-45 cells were exposed to various forms of TiO2, and cell viability at 24, 48, and 72 hours with different concentrations of nanoparticles was evaluated using MTT [Figure 1]. In the PEG-amorph TiO2 nanoparticles group, increasing concentration and exposure time led to an increase in cell toxicity (p ≤ 0.050). For PEG-amorph TiO2, the most toxic effect of nanoparticles was at concentrations of 50 μg/mL at 24, 48 and 72 hours. In the amorph TiO2 group, nanoparticle toxicity was the highest at a concentration of 30 μg/mL for 72 hours. In the PEG-brookite group, the highest toxicity levels on cancer cells were observed at 40 μg/mL for 72 hours. In rutile, anatase, amorph BSA and brookite BSA groups, the highest toxicity to cancer cells was at a concentration of 50 μg/mL nanoparticles for 72 hours.
Nanoparticles caused cell death in the MKN-45 cancer cells. In previous studies, nanoparticles caused cell death by activating the intrinsic apoptotic pathway.16 Apoptosis in MKN-45 cells was tested using AO/EB staining [Figure 2]. After exposure to TiO2 nanoparticles, we saw cell death (p ≤ 0.050).
In all cases, the nanoparticles concentration was 50 μg/mL and MKN-45 cells were exposed to nanoparticles for 48 hours. The percentages of apoptotic and live cells in the control group were 10.0% and 90.0%, respectively, and the percentages of apoptotic and live cells following exposure to TiO2 nanoparticles are given in Table 1.
Table 1: The percentage of apoptotic and live cells following exposure to titanium oxide (TiO2) nanoparticles.
|
Control |
10.0 |
90.0 |
|
PEG-amorph TiO2 |
40.0 |
60.0 |
|
Amorph TiO2 |
75.0 |
25.0 |
|
PEG-brookite |
72.0 |
28.0 |
|
Rutile |
75.0 |
25.0 |
|
Anatase |
40.0 |
60.0 |
|
Amorph BSA |
80.0 |
20.0 |
|
Brookite BSA |
85.0 |
15.0 |
PEG: polyethylene glycol; BSA: bovine serum albumin.
Invasion of cancer cells after exposure to nanoparticles was evaluated using wound healing assay. We saw an increase in cell invasion in the PEG-amorph TiO2 group compared to the control group, and a decrease in cell invasion in the brookite BSA group (p ≤ 0.050). Our results suggest that different configurations of nanoparticles have different effects on cancer cell invasion. We saw no significant changes when using amorph TiO2, PEG-brookite, rutile, anatase, and amorph BSA nanoparticles [Figure 3].
Nanotechnology has been introduced as a new tool for the treatment of diseases like cancer.21 Nanomaterials can be used for cancer detection and imaging.22 Nanomaterials are used as vector for drug delivery for cancer treatment.23 For example, gold nanoparticles can be implemented as drug carriers in cancer treatment.24 Nanomaterials are also used in gene therapy as a carrier for DNA and RNA.25
Some nanomaterials selectively target cancer cells. For example, zinc oxide nanoparticles (ZnO) have toxic effects on human myeloblastic leukemia cells (HL60) more than normal peripheral blood mononuclear cells. This is a therapeutic benefit for using nanomaterials in cancer treatment since common chemotherapy drugs cannot distinguish cancerous and healthy cells and causes side effects. ZnO nanoparticles induce apoptosis in cancer cells by creating free oxygen radicals.26
The authors declared no conflicts of interest. No funding was received for this study.
We would like to thank the deputy of research and student research committee of Semnan University of Medical Sciences for providing facilities to this work.