|
Abstract
Objectives: Currently recommended risk stratification protocols for suspected ischemic chest pain in the emergency department (ED) includes point-of-care availability of exercise treadmill/nuclear tests or CT coronary angiograms. These tests are not widely available for most of the ED’s. This study aims to prospectively validate the safety of a predefined 4-hour accelerated diagnostic protocol (ADP) using chest pain, ECG, and troponin T among suspected ischemic chest pain patients presenting to an ED of a tertiary care hospital in Oman.
Methods: One hundred and thirty-two patients aged over 18 years with suspected ischemic chest pain presenting within 12 hours of onset along with normal or non-diagnostic first ECG and negative first troponin T (<0.010 μg/l) were recruited from September 2008 to February 2009. Low-probability acute coronary syndrome (ACS) patients at 4-hours defined as absent chest pain and negative ECG or troponin tests were discharged home and observed for 30-days for major adverse cardiac events (MACE) (Group I: negative ADP). High-probability ACS patients at 4-hours were defined by recurrent or persistent chest pain, positive ECG or troponin tests and were admitted and observed for in-hospital MACE (Group II: positive ADP).
Results: One hundred and thirty-two patients were recruited and 110 patients completed the study. The overall 30-day MACE in this cohort was 15% with a mortality of less than 1%. 30-days MACE occurred in 8/95 of group I patients (8.4%) and 9/15 of the in-hospital MACE patients in group II. The ADP had a sensitivity of 52% (95% CI: 0.28-0.76), specificity of 93% (0.85-0.97), a negative predictive value of 91% (0.83-0.96), a positive predictive value of 60% (0.32-0.82), negative likelihood ratio of 0.5 (0.30-0.83) and a positive likelihood ratio of 8.2 (3.3-20) in predicting MACE.
Conclusion: A 4-hour ADP using chest pain, ECG, and troponin T had high specificity and negative predictive value in predicting 30-day MACE among low probability ACS patients discharged from ED. However, 30-day MACE in ADP negative patients was relatively high in contrast to guideline recommendations. Hence, there is a need to establish ED chest pain unit and adopt new protocols especially adding a point-of-care exercise treadmill test in the ED.
Keywords: Emergency department; Accelerated diagnostic protocol; Acute coronary syndrome; MACE; Exercise treadmill test; Chest pain unit.
Introduction
Triage and management of patients with suspected ischemic chest pain in the emergency department (ED), who have a low probability acute coronary syndrome (ACS) is challenging. Current guidelines suggest that patients with suspected ischemic chest pain should undergo rapid assessment with repeat ECGs and serial cardiac injury markers.1 There are many emergency triage protocols/algorithms/risk scores (e.g. Thrombolysis in Myocardial Infarction [TIMI] risk score) using a variety of variables to risk-stratify chest pain patients in the ED.2-4 Even though, these risk scores have high sensitivity in diagnosing ACS they do not have adequate specificity to discharge low-risk patients safely and they are not widely applied in the ED. In addition, guidelines recommend chest pain units (CPU) with point-of-care use of exercise treadmill test (ETT) or nuclear scan as well as computed tomography coronary angiography (CTCA) before discharging low-risk patients from ED.1 These modalities are not widely available in ED especially in developing countries including Oman. A 4-hour risk stratification accelerated diagnostic protocol (ADP) using three variables chest pain, ECG and Troponin T is being followed in our ED to identify low probability ACS with early discharge of patients. The aim of this study was to prospectively validate the safety of a predefined 4-hour ADP using chest pain, ECG, and troponin T among patients with suspected ischemic chest pain presenting to ED.
Methods
One hundred and thirty-two patients aged over 18 years with suspected ischemic chest pain lasting more than 5 minutes and presenting within 12 hours onset along with normal or non-diagnostic ECG and negative first Troponin T were recruited. The study was conducted from September 2008 to February 2009 at Royal Hospital, Muscat, Oman. Troponin T was analyzed using 4th-generation Roche Elecsys Troponin T assay done in the laboratory. A positive troponin T was defined as >99th percentile of the assay used (>0.01 μg/l). Ethical approval was obtained and all patients gave informed consent.
The inclusion criteria was based on the following factors: 1). Chest pain or discomfort suspected to be ischemic and lasting more than 5 minutes but within 12 hours duration; 2). ECG: normal or non diagnostic (nonspecific changes or unchanged from previous); and 3). Troponin T negative on arrival.
The exclusion criteria was based on the presence of the following factors: 1). Age <18 years; 2). High probability ACS features on arrival- On going chest pain with or without new ischemic ECG changes (ST-segment elevation or depression ≥1 mm or T-wave inversion >2 mm in ≥2 anatomically contiguous leads), positive Troponin T, or hemodynamic compromise, were directly admitted, and excluded from the study; 3). Any other clear cause other than ACS for the symptoms; 4). ACS in past 4 weeks; 5). ECG showing left bundle branch block or pacing rhythm; 6). Chronic kidney disease requiring dialysis; and 7). Anemia requiring transfusion.
In accordance with the American Heart Association (AHA) case definitions, possible cardiac symptoms included acute chest, epigastric, neck, jaw, or arm pain, or discomfort or pressure without an apparent non-cardiac source.5 More general, atypical symptoms (fatigue, nausea, vomiting, diaphoresis, faintness, and back pain) were not used as inclusion criteria in the absence of chest pain. ECG changes were defined according to American College of Cardiology (ACC)/AHA criteria.6
|
Step 1: Identify patients with suspected ischemic chest pain + normal or non diagnostic ECG (nonspecific changes or unchanged from previous) + Troponin T negative on arrival.
Step 2: Persistent or recurrent chest pain, or new ECG changes or troponin test positive at 4-hours; admit to coronary care unit. N.B. for patients presenting within 2 hours onset of chest pain, the protocol is extended to 6 hours.
Step 3: Negative step 2, admit patient to ED based chest pain unit for ETT. If ETT positive, admit coronary care unit or monitored bed.
Step 4: If ETT negative; discharge patient with cardiology clinic appointment within 30 days.
|
Figure 1: The New 4-hour Accelerated Diagnostic Protocol.
The main outcome measures are based on either of two options:
1). Primary end point - Major adverse cardiac events (MACE) including death, myocardial infarction, and revascularization at 30 days for patients discharged and in-hospital MACE for those admitted; and 2). Secondary end point - Follow-up exercise stress test (ETT or nuclear) results among patients discharged from ED.
All patients had an ECG and Troponin T within 10 minutes of arrival and at four hours. For patients who presented less than two hours after the onset of chest pain, ECG/Troponin T was performed six hours after the onset of pain, so that tests were performed in all patients at least six hours after the onset of pain. Data collected included demographic, clinical, ECG, Troponin T, and outcome data including MACE. At 4 hours, low probability ACS patients defined as "no chest pain, negative ECG and Troponin T tests" were discharged home with 30-day clinic appointment. All parameters had to be negative at 4 hours for the ADP to be considered negative (and thus for the patient to be identified as low probability ACS). High probability ACS patients at four hours defined as "presence or absence of chest pain with or without positive ECG or Troponin T test" were admitted and followed for MACE in-hospital. Any of the three parameters if positive was considered high probability ACS. They underwent in-hospital coronary angiography and revascularization as per hospital practice.
Discharged patients were later seen in cardiology clinic at 30-days to know any MACE. MACE was confirmed by reference to hospital notes or other hospital/GP records. ETT or nuclear scan was done on clinic patients for further stratification. The criteria for MACE included: death, myocardial infarction [ST-elevation myocardial infarction (STEMI) and non-STEMI) and any revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass graft surgery [CABG]). In the event of undetermined cause of death, this was assumed to be cardiac. Definition of clinical variables and myocardial infarction was based on ACC/AHA guidelines.6
Data are presented as number (%) and/or mean ± SD. Patients were divided into two groups: Group I Low-probability ACS with negative ADP and Group II High-probability ACS with positive ADP. The Fisher’s exact test and unpaired t-tests were used to compare baseline variables between two groups. P values less than 0.05 (two-tailed) were considered to indicate statistical significance. χ2 analyses were used to generate two-by-two tables for the calculation of sensitivity, specificity, positive/negative predictive values, and likelihood ratios. All analyses were done with SPSS 10.
Results
132 consecutive patients were recruited and 110 patients completed the study. Patients who went against medical advice and those who did not visit follow-up clinic were excluded from the analysis. There were 95 patients in the ADP negative group and 15 patients in the ADP positive group. 71% of the patients were men in Group I and 66% in Group II. There was no difference in age between groups I and II (52 ± 13 vs. 55 ± 9; p=0.39). Other cardiovascular risk factors and background cardiovascular past medical history were statistically similar between the two groups. (Table 2)
There was no difference in length of ED stay between groups I and II (5.07 ± 0.74 vs. 5.44 ± 0.82 hours). About 30-40% of the patients presenting to ED with chest pain had past CAD with significant number of PCI and CABG patients.
Table 1: Baseline characteristics of low probability (ADP negative) and high probability (ADP positive) ACS patients with suspected ischemic chest pain presenting to emergency department.
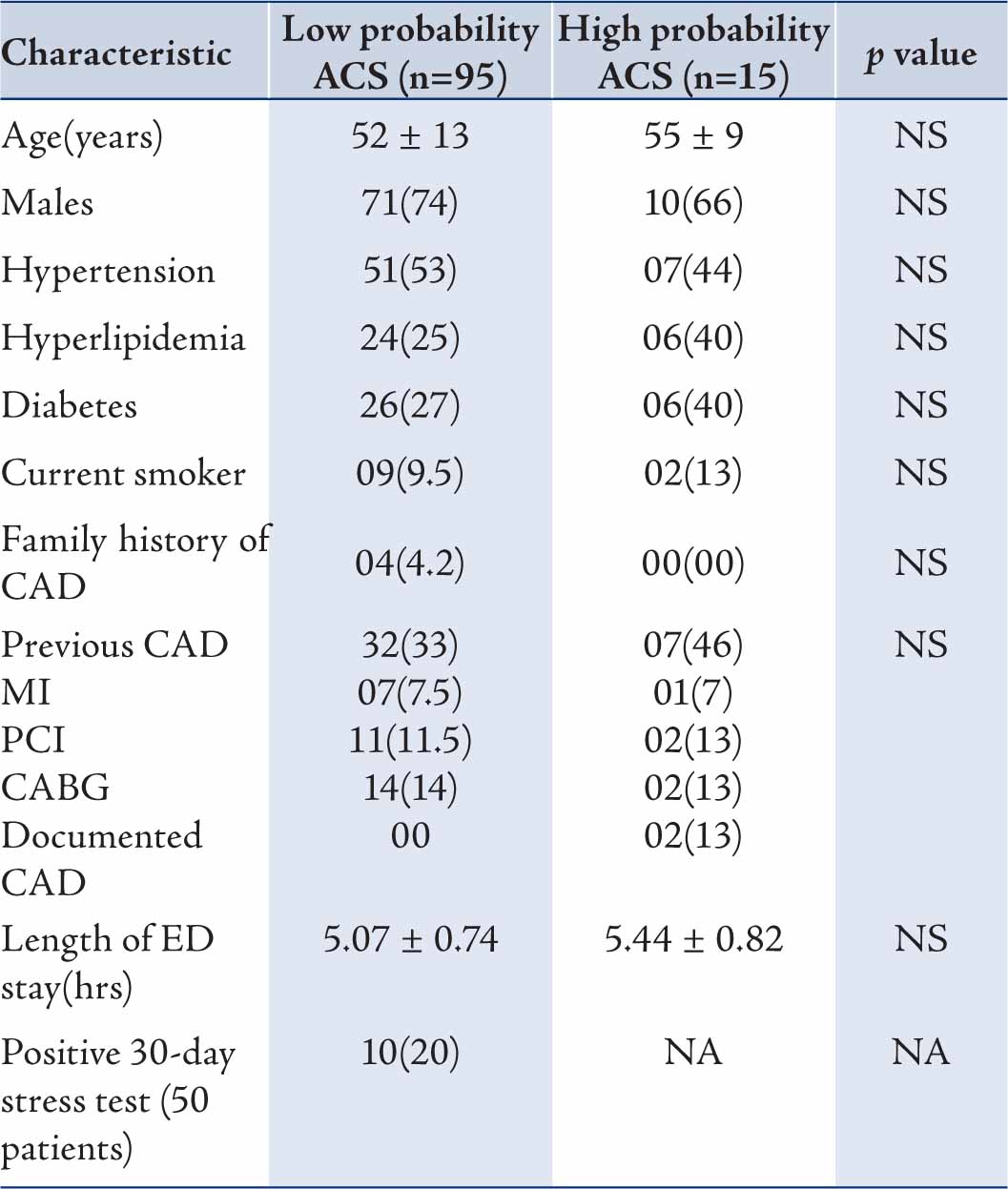
Values are n (%) or mean ± SD unless specified; ADP=Accelerated diagnostic protocol; ACS=Acute coronary syndrome; CAD=Coronary artery disease; MI=Myocardial infarction; PCI=Percutaneous coronary intervention; CABG=Coronary artery bypass graft surgery; ED=Emergency department; NS=Not significant; NA=Not applicable.
Table 3 shows MACE in the two groups. Total MACE was 15% (17/110). MACE occurred in 8 (8.4%) and 9 (60%) patients in Groups I and II, respectively. There was one death (1.05%) in the ADP negative group along with 1 ST-elevation MI (1.05%), 3 CABG (3.15%) and 3 PCI (3.15%) procedures within 30-days follow up. The patient who died was a 70 year old male who presented 25 days after discharge from ED in cardiac arrest and expired after unsuccessful resuscitation.
In the ADP positive group, there was no death, 3 (20%) patients had non-STEMI and 6 patients underwent revascularization [2 (14%) PCI and 4 (26%) CABG]. Remaining 6 patients had either normal coronary arteries or non significant CAD on coronary angiography. All these six patients had normal ECG and troponin T at 4 hours and were admitted based on chest pain and treated as unstable angina. Only 50 of the 95 patients discharged had an ETT or nuclear scan. Among these 50 patients 10 (20%) patients tested positive at 30-days indicating underlying CAD.
The ADP had a sensitivity of 52% (95% CI: 0.28-0.76), specificity of 93% (0.85-0.97), a negative predictive value (NPV) of 91% (0.83-0.96), a positive predictive value (PPV) of 60% (0.32-0.82), negative likelihood ratio (LR) of 0.5 (0.30-0.83) and a positive LR of 8.2 (3.3-20) in predicting MACE (Table 4). NPV was high at 91% indicating that in a patient with ADP negative result there is 91% chance that he/she does not have short-term MACE; leaving 9% chance that it is false negative. Similarly, if ADP is positive, there is 60% chance that patient has MACE; leaving 40% chance that it is false positive.
Table 2: 30-days MACE in patients with negative ADP and in-hospital MACE in patients with positive ADP among suspected ischemic chest pain patients presenting to emergency department.
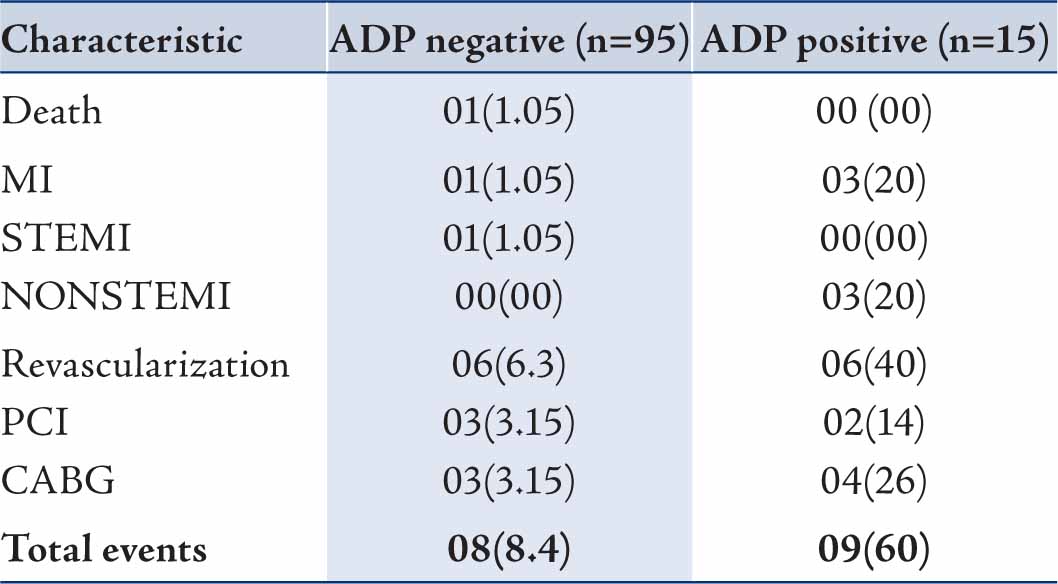
Values are n (%); MACE=Major adverse cardiac events; ADP=Accelerated diagnostic protocol; MI=Myocardial infarction; STEMI=ST-elevation myocardial infarction; PCI=Percutaneous coronary intervention; CABG=Coronary artery bypass graft surgery.
Table 3: Accuracy (95% Confidence Interval) of an accelerated diagnostic protocol for prediction of MACE in patients with suspected ischemic chest pain presenting to an emergency department.
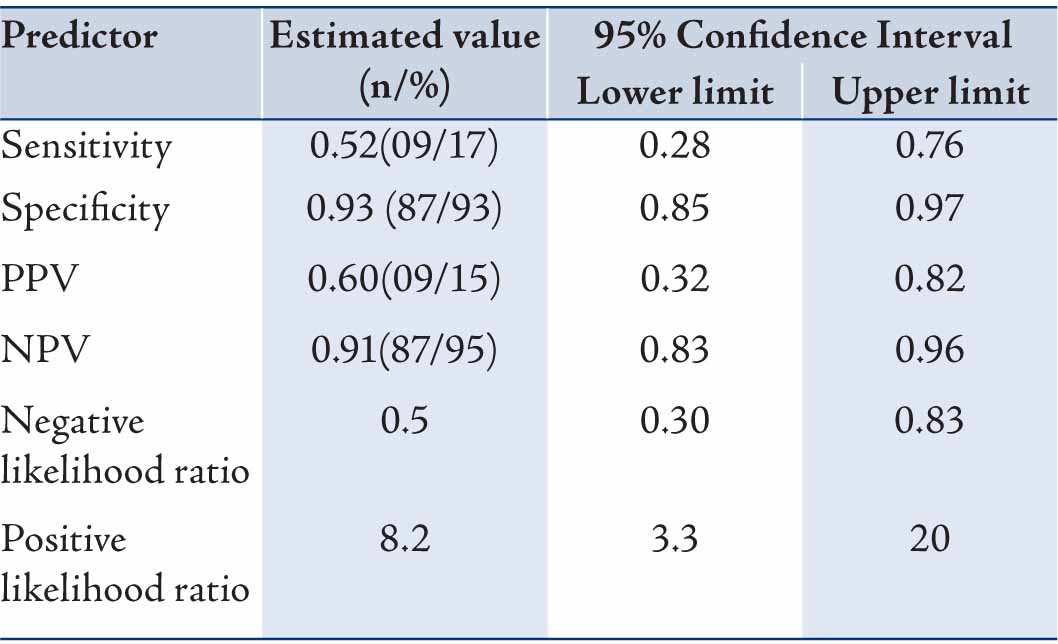
Values are n or %; MACE=Major adverse cardiac events; PPV= Positive predictive value; NPV=Negative predictive value
This study from a tertiary hospital in Oman has prospectively validated that a 4-hour ADP using chest pain, ECG, and troponin T can discharge low probability ACS patients from the ED with high specificity and NPV for short-term MACE. However, the incidence of MACE at 30-days was relatively high at 8.4% in contrast to guideline recommendations which aim for less than 2% MACE at 30-days.1
US data suggest that failure to detect ACS resulting in inappropriate discharge of such patients from the ED may exceed 4%, with a risk-adjusted mortality ratio that is nearly 2-fold that of patients hospitalized for ACS.7 Selker et al. developed an electronic tool called the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) based on the clinical and ECG findings which increased the rate of appropriate discharges by unsupervised residents with less than 10% risk of ACS.8 In a study, Limkakeng et al. reported a 30-day MACE of 4.9% among chest pain patients who were categorized as low risk and discharged from the ED.9 A Canadian study showed 4.6% and 6.4% missed cases of acute myocardial infarction and unstable angina, respectively discharged from the ED.10 However, recent studies have shown less incidence of MACE at 30-days. In a study of 2271 patients presenting to the ED with chest pain, a low-risk group with a 30-day MACE of 2.5% was recognized.2 In a recent meta-analysis of 10 studies assessing TIMI risk score, there was 1.8% incidence of MACE at 30-days in low-risk patients discharged home.11 In another study a 2-hour ADP using TIMI score, ECG, and 3 biomarkers showed 0.9% incidence of MACE at 30-days.12
Increasing research is emerging into the use of ADP incorporating confirmatory testing in the chest pain unit (CPU).1 The main aim of confirmatory testing which includes ETT or nuclear scans is to further minimize the likelihood of ACS to a level so low that discharge is safe.1 These protocols typically include the use of serial biomarkers and ECG’s for 6-12 hours, if negative, ETT was performed.1,13,14 During follow-up of 1 to 17 months in the above studies, there was 1 cardiac death, and the incidence of MACE was 0% to 2%, reflecting a very high NPV for MACE after ETT in CPU patients. No complications of early ETT were reported. However, PPV is low, but the number of unnecessary admissions is reduced. In a study involving 175 patients, after ETT in the ED 113 patients were discharged, and 62 were admitted. ETTs PPV for coronary artery disease among admitted patients were 35.7%, and sensitivity was 95.2%. ETTs NPV among discharged patients were 99.1%. None of the 113 discharged patients returned to the ED for cardiac reasons during the 30-day follow-up period.15
The current ACC/AHA guidelines for stress testing and for management of non-ST-segment-elevation ACS recommend that ETT without imaging should be performed as the initial test in low-to-intermediate-risk patients who present with ischemic symptoms and can exercise, do not have significant baseline ECG changes that preclude interpretation (0.05 mV ST-segment depression, left ventricular hypertrophy with any repolarization abnormality), and are not taking digoxin.6
Alternatively, myocardial imaging with stress myocardial perfusion imaging (MPI) or echocardiography is more accurate in detecting CAD than ETT. Stress MPI and stress echocardiography have a sensitivity and specificity of 87% and 73% and 86% and 81%, respectively for detecting obstructive CAD.1,16,17 However, it has been noted that both ETT and stress imaging methods have comparable NPVs for ACS when used in the ED among low-risk ACS patients.1 Hence stress MPI or echocardiography may be used if available especially in patients who are unable to do treadmill exercise or with baseline ECG changes. Rest MPI (if available) has a class 1 indication in current guidelines for evaluation of patients with chest pain and a nonischemic ECG.17 With regard to rest echocardiography, a more recent study in patients admitted with symptoms suggestive of ACS revealed a NPV of 97% but a PPV of only 24%.18 These latter results are comparable to those of ETT in a similar patient population. Recently, CTCA has emerged as a point-of-care test to rule out ACS in the ED. A larger single-center study of 368 patients presenting to the ED with chest pain, CTCA revealed a sensitivity of 100% and a negative predictive value of 100% for ACS/MACE after 6 months of follow-up.19
Although from this study it can be concluded that majority of the patients who are ADP negative have a good prognosis and can be discharged early from ED; the drawback was the incidence of MACE that was relatively high at 30-days. In addition, this protocol resulted in 40% false positive patients who were admitted. This was predominantly due to chest pain component of the protocol which is subjective.20 In a meta-analysis to identify the elements of the chest pain history that may be most helpful to the clinician in identifying ACS in patients presenting with chest pain, it was noted that although certain chest pain characteristics decrease or increase the likelihood of ACS, with negative and positive likelihood ratios that range from 0.2 to 4.7, respectively; none of them were powerful enough to support discharging patients according to the chest pain history alone.20 Hence, in realty many patients are admitted if chest pain is persisting even though it may not indicate ACS.
This protocol reduces the number of patients needing prolonged assessment in the ED as well as prevents overcrowding. However, to reduce number of false negative patients and decrease MACE further, as well as reduce false positive patients and decrease unnecessary admissions, it is prudent to adopt point-of-care ETT in the ED. Another option is to adopt TIMI risk score, but recent studies have shown that the specificity is so low; another confirmatory test is needed in addition to risk score.21,22 Hence adding ED ETT to this present protocol is more appropriate. Immediate ETT in the CPU in the ED for low risk patients is feasible, inexpensive, safe, and accurate for determining those who can be discharged safely from the ED. Further studies are needed to evaluate the accuracy of ETT incorporated ADP in this population. The ESCAPE (effectiveness and safety of chest pain assessment to prevent emergency admissions) trial from UK showed that CPU care was more cost effective along with a low 30-day MACE of 1.7% in those patients discharged from CPU.23,24 Fig. 1 shows the suggested new ADP protocol.
The study has few limitations. The present study included a convenience sample of eligible patients presenting at the time when the primary investigator was either working or present in the ED, spread across 24 hrs. The entire population at risk over the time period was not studied. The follow-up was limited to 30 days only. Moreover, not all patients discharged had an ETT. Newer troponin T assays, which typically have lower detection limits and higher analytical precision, would probably improve the sensitivity of this ADP for the prediction of MACE.
Conclusion
A 4-hour ADP using chest pain, ECG, and troponin T had high specificity and negative predictive value in predicting 30-day MACE among low probability ACS patients discharged from ED. However, 30-day MACE in ADP negative patients was relatively high in contrast to guideline recommendations. Hence, there is a need to establish ED chest pain unit and adopt new protocols especially adding a point-of-care exercise treadmill test in the ED.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
|