| |
Abstract
Objective: This study was aimed at providing an analysis of the correlation between CD4/CD8 counts and some coagulation factors in HIV-Positive Iranian patients.
Methods: A case-control study on 58 HIV-infected patients and control group (58 healthy individuals). Patients and controls were matched for sex and age. In this study, several blood parameters were measured in 58 HIV-infected patients and the controls. Laboratory data were then measured including hemoglobin, platelets, homocysteine, serum levels of IgM and IgG antiphospholipid antibodies (aPL), IgM and IgG anticardiolipin antibotdies (aCL), and CD4+ and CD8+ cell count.
Results: The HIV-infected patients, compared to healthy controls, showed a significant decline in platelets, CD4 count and CD8 count (p<0.0001), and an increase of homocysteine (p<0.0001) and IgG aPL levels (p<0.0001). No statistical difference was found between patients with CD4 count £200 and CD4 count >200 in the evaluated variables.
Conclusion: The results showed that thrombophilic abnormality in the form of hyperhomocysteinemia is more frequent in HIV-infected patients and should be considered by clinicians in view of an early diagnosis of the hypercoagulability state to prevent thrombotic complications.
Keywords: CD4/CD8 Counts; Coagulation Factors; HIV-Positive Patients.
Introduction
Human immunodeficiency virus (HIV) infection may result in variable hematologic manifestations.1 Several clinical studies have reported a higher risk of thrombotic complications among HIV-infected patients.2-4 The risk of venous thrombotic events has been described 6.5 times,3 and 10 times,5 more prevalent in these patients than in general population, and autopsy studies have shown high frequencies of previously undiagnosed thromboembolism among patients with AIDS.6 HIV patients with CD4 counts <200 cells/μL have a greater risk of thrombosis than those with higher CD4 counts.7 A wide variety of coagulation abnormalities could predispose to a hypercoagulable state in HIV disease, including the high levels of antiphospholipid antibodies and anticardiolipin antibodies, deficiencies of protein C, protein S,8,9 antithrombin III and heparin factor II,8 increased platelet activation and an increase of homocysteine levels.8,9 The early diagnosis of these abnormalities could prevent potentially lethal thrombotic complications including pulmonary thromboembolism. Hence, in this study, the presence of some thrombophilic abnormalities in HIV-infected patients was evaluated and compared to HIV negative comparators. A second objective of this study was to examine the association between CD4 count and coagulation abnormalities in HIV-infected patients.
Methods
In this case-control study investigsting 58 HIV-infected patients (HIV infected cases treated naive) who visited the outpatient clinic of our teaching hospital (the HIV positives had been presented one year after they were diagnosed with their disease), Tehran University Medical Sciences, Iran.
HIV infection was documented by serology, confirmatory western blot analysis or by polymerase chain reaction (PCR). The control group included 58 healthy individuals who attended the biochemical laboratory of the hospital to undergo periodical checkup. Patients and controls were matched in age and sex. The study was approved by the hospital’s ethics committee.
Whole blood, serum or plasma were collected from all subjects and eight markers were measured: Hemoglobin and platelets measured by cell counter (sysmex, KX21- Japan); Homocysteine measured by auto-analyzer (Alcion-300, Abott-USA), Turbidometric method; Anticardiolipin and Antiphospholipid antibodies (IgG, IgM) measured by ELISA method and lymphocyte CD4, CD8 counts were done by a single platform flow cytometric method.
Anticardiolipin antibodies (aCLs) (IgG, IgM) were measured using ELISA (ORGENTEC Diagnostika Gmbh, Germany kits). Normal values were <10 U/ml for IgG Ab and <7 U/ml for IgM Ab. The lower detection limit for Anticardiolipin IgG was determined at 1 GPL U/ml. Anticardiolipin IgM yielded a sensitivity of 0.5 MPL U/ml. Antiphospholipid antibodies (IgG, IgM) were measured by (ELISA) ORGENTEC Diagnostika Gmbh, Germany Kits. Normal values were <10 U/ml for IgG, IgM Abs. The lower detection limit for Antiphospholipid screen IgG and IgM were determined at 0.5 U/ml.
Serum Homocysteine was measured by Diazyme Laboratories, USA Kit. Normal value for Homocysteine was 5-18 μmol/L. Anemia was defined as a hemoglobin level <12 g/dL, based on standard published guidelines. Platelet number <150 × 10³/μL was considered as thrombocytopenia.
The SPSS-for-Windows V 16.0 was used for data analysis. The distribution of the quantitative data was examined by One-Sample Kolmogorow-Smirnov Test. In order to analyze the association between quantitative variables, independent samples t-test and the Mann-Whitney U test were used, depending on the normality of data. Chi-square test and Fisher’s exact test were used for the qualitative data. Pearson´s correlation coefficient obtained to evaluate the correlation between the coagulation factors and CD4 count. A two-tailed p-value of less than 0.05 was considered statistically significant.
Results
In this case-control comparative study, 38 (65.5%) men and 20 (34.5%) women were enrolled in each group. From our data, CD4+ cell count was 327.55 ± 196.54 in HIV infected patients compared to 920.26 ± 168.42 in the controls (p=0.00010). In addition, CD8+ cells count was 803.93 ± 332.98 in HIV infected cases compared to 965.09 ± 111.54 in the controls (p=0.00011), which were both significantly different. The serum levels of all classes of antibodies including IgG and IgM aPL, and IgG and IgM aCL were 3.17 ± 1.46 and 2.62 ± 1.68, and 1.99 ± 0.84 and 1.56 ± 1.03, respectively in patients, all of which were within the normal range. However, the mean levels of Ig classes were as follows: 1.92 ± 1.04 for IG aPL; 2.23 ± 1.37 for IgM aPL; 1.92 ± 1.23 for IgM aCL; and 1.91 ± 1.14 for IgG aCL; among these, only IgG aPL exhibited a statistically significant difference (p=0.00010). There was no significant difference in the mean values of IgM aPL, IgG aCL and IgM aCL between cases and comparators. Clinical characteristics of the subjects evaluated in this study are shown in Table 1.
Hyperhomocysteinemia was found in 53 (91.4%) of 58 patients compared with the controls with normal homocysteine and the levels of homocysteine were significantly higher in patients compared with the controls. A platelet count below 150 × 103/L was detected in 13 patients (22.4%) vs. 1 (1.7%) of the comparators and the mean platelets was significantly lower in patients compared to the controls (p=0.00010).
Anemia was detected in 5 patients (8.6%) vs. none of controls. There was no significant difference in the mean values of hemoglobin between cases and controls. Patients with CD4 count £200 and CD4 count >200 did not show significant difference in any variables. (Table 2)
Table 1: Clinical characteristics of HIV-infected patients and controls.
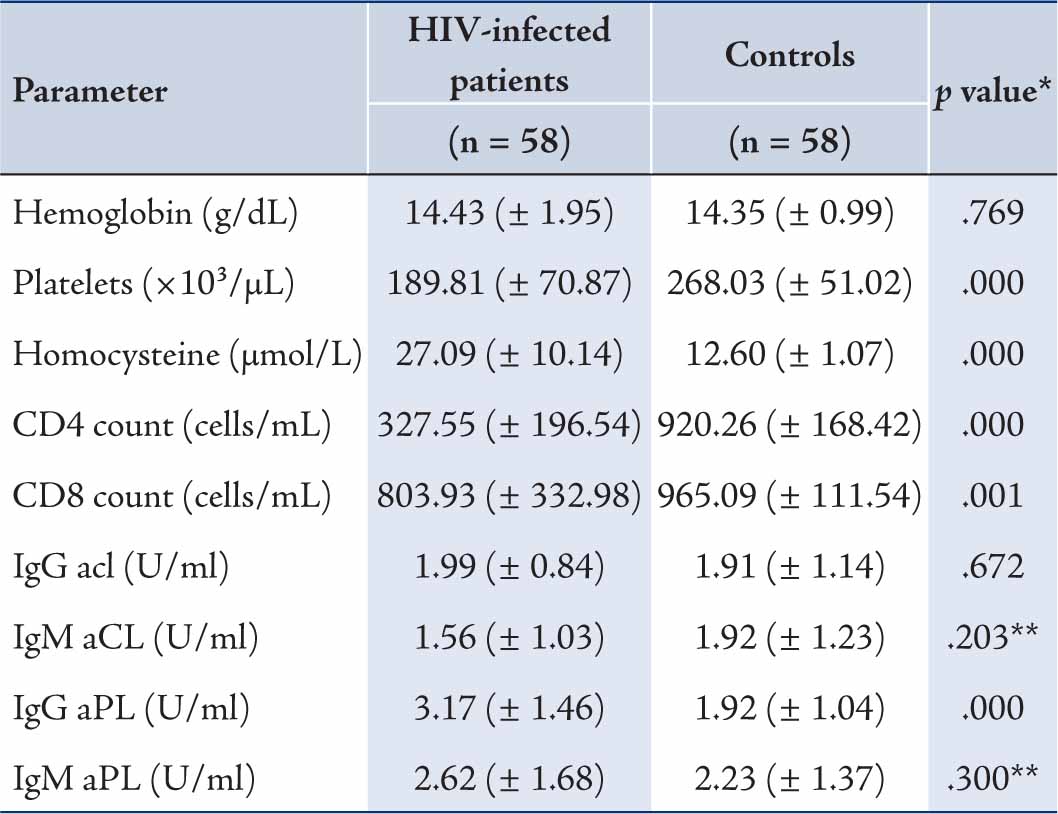
* p value were computed using Independent Sample t-test
Table 2: Mean values of evaluated parameters in CD4 £200 and CD4 >200 among HIV-infected patients.
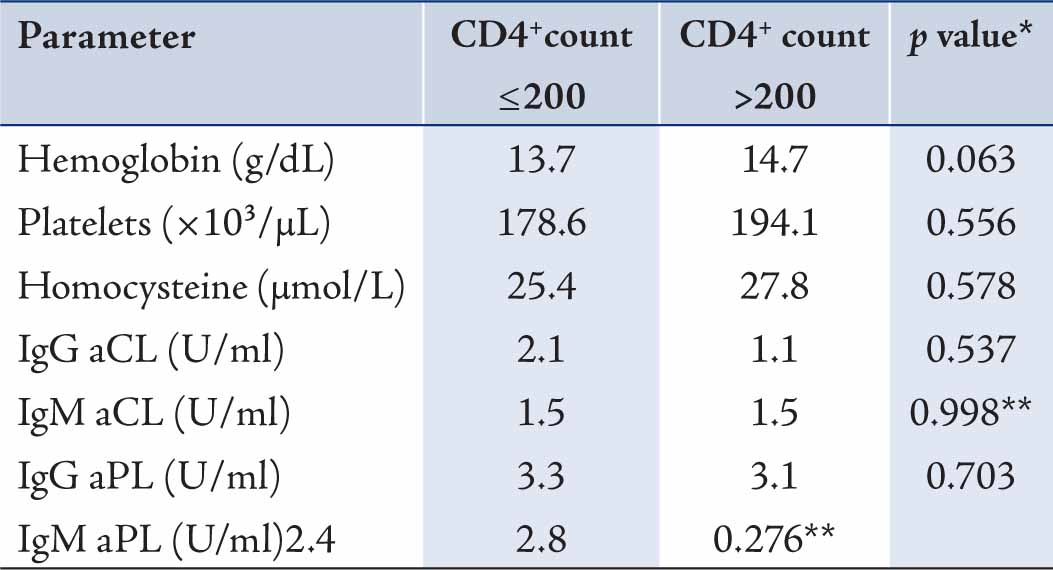
* p value were computed using Independent Sample t-test
Two parameters were shown to be correlated with CD4 count among HIV-infected patients. There was a moderately strong positive correlation between hemoglobin and CD4 count (r=0.31; p=0.018) and between platelet count and CD4 count (r=0.27; p=0.041). No statistical correlation was observed between CD4 count and the levels of homocysteine, IgG aCL, IgM aCL, IgG aPL and IgM aPL in the studied patients.
Discussion
Various hematological abnormalities could predispose to a hypercoagulable state and an increased incidence of thrombotic events in HIV infection. Several studies have proposed the participation of antiphospholipid antibodies (aPL) in the progression of thrombotic complications in HIV patients.8-10 These antibodies are highly prevalent in HIV infection, reported in 82% to 92% of patients with AIDS.11 Both types of aPL have been found in HIV patients.12 Although, IgG aPL were more prevalent (41%) in HIV infection than IgM aPL (7%).12All of our patients had normal values of IgG aPL (1.3 - 7.3 U/mL) and IgM aPL (0.6 - 9.8 U/mL), although the mean values of IgG aPL levels in patients were significantly higher than the controls (p=0.00010), confirming previous studies on the matter. From the current data, it appears that there is no correlation between the severity of the disease (presented as CD4+ cells count) and an increase in the levels of aPL.
Anticardiolipin antibodies (aCL) have been detected in almost 45% to 50% of HIV-infected patients.11,12 Maclean et al. found aCL in a large number of these patients and the levels were significantly higher than among the controls.13 Sorice and colleagues reported a high prevalence of IgG aCL in HIV-infected patients (77.1% of 35 patients) that was significantly higher than HIV negative controls (p<0.0001).14 Guerine et al. observed the increased levels of aCL in 88% of 30 HIV patients.15 In contrast to these findings, we found no significant difference in the levels of aCL between patients and controls. This discrepancy may be due to the small number of sample population, methodological reasons, laboratory methods or genetic differences of study population. Moreover, we observed no correlation between aPL and CD4 count. In 2003, a case-control study by Chretien and colleagues showed that IgG and IgM aCL levels were statistically more frequent in 105 HIV patients than 100 sex- and age-matched healthy controls. They found no correlation between these antibodies and CD4 count.16 In 2007, a relatively high frequency of aCL antibodies in HIV (17.8% in 90 patients) with 16.7% positive for IgG aCL and 3.3% for IgM aCL reported by Galrão et al. In their study, no association was observed between CD4 count <200 and aCLs.17 The recent findings of two previous studies were in agreement with our results.
Hyperhomocysteinemia was found in 91.4% of our patients versus none of the controls and the plasma homocysteine levels were significantly higher in patients as compared with control subjects (p=0.00010). The increase in serum hemosisteine of HIV patients may have resulted from special antibodies in the serum or their immunity shortcomings. The association between cardiovascular risk and increased plasma homocysteine levels has been reported. The prevalence of hyperhomocysteinemia in HIV-positive patients has been reported to be between 12.3% and 35%.9 In 2001, Bernasconi et al. found significantly higher fasting plasma homocysteine levels in 82 HIV patients who received highly active antiretroviral therapy in comparison with 80 healthy controls.9 Such a high level of homocysteine has been correlated with nutritional deficiency and antiretroviral therapies.
Considering the high prevalence of hyperhomocysteinemia in our patients, we suggest regular examinations of cardiovascular system in HIV-infected patients to prevent atherothrombotic vascular complications and to reduce cardiovascular risk. On the other hand, HIV-infected patients have an increased risk of hemorrhagic complications by reason of decreasing platelet count. Thrombocytopenia is one of the first clinical signs of HIV infection. As shown in other studies, thrombocytopenia developed in approximately 10-40% of HIV-infected patients.18 Although the average platelets volume in both groups stands at the normal range, it is lower in HIV-infected group than in HIV-negatives.
In 2003, Palomo et al. observed thrombocytopenia in 16.2% of 37 HIV patients.19 Our study showed that 21% of patients had the platelet count <150 × 10³/dL and the mean values of platelets in patients was significantly lower than in the controls. Moreover, we found a moderately strong positive correlation between the number of platelets and CD4 count (r=0.27; p=0.041), in an agreement with Elaine et al.20 In a large prospective study, Kaslow and colleagues compared 1,611 HIV-positive patients in hematologic abnormalities with 2,646 controls and observed that 6.7% of patients presented thrombocytopenia. They found an inverse relationship between the intensity of thrombocytopenia and CD4 counts, confirming our findings.21 However, none of the HIV infected patients in the current study was defined as thrombocytopenic nor did they show any symptoms of this disorder. By reason of the high frequency of thrombocytopenia in HIV patients as already discussed, they should be examined for platelet count even before minor surgeries to prevent hemorrhagic complications.
Anemia is a frequent complication of HIV infection. Ramezani et al. observed that mild to moderate anemia existed in 46% of 143 HIV infected subjects.22 We observed no significant difference in hemoglobin levels between patients and controls (p=0.769). This finding may be attributable to the number of subjects included or the supplementary treatment with Iron or folate-vitamin B12 complex. We found that there is a moderately strong positive correlation between the hemoglobin levels and CD4 count (r=0.31; p=0.018) in the current studied patients. Low CD4 counts (<200 cells/ml) have been associated with an increased risk of anemia.23 In a large cohort of HIV-infected women in 2001, an inverse correlation between CD4 count and the risk of anemia was found by Levine et al.24 In 2007, Mildvan et al. reported a high prevalence of anemia in 9690 HIV-infected patients and a strong correlation between anemia and low CD4 count.25 Recently, a prevalence of 20% was reported for anemia in 63 HIV patients by Mata-Marin and colleagues. Also, they detected a moderately strong positive correlation between CD4+ cell count and hemoglobin levels (r=0.595; p<0.0001) that confirms our findings.
The main limitations to the current study include its cross-sectional design, the limited number of subjects included and the lack of adequate information regarding clinical evidence of thrombotic events in patients. Further studies are needed to evaluate the association between such thrombophilic abnormalities and the occurrence of thrombotic events in HIV patients.
Conclusion
Our results showed that some thrombophilic abnormalities are more frequent in HIV-infected patients compared to HIV-negative controls and should be considered by clinicians in view of an early diagnosis of the hypercoagulability state to prevent thrombotic complications including pulmonary thromboembolism.
Acknowledgments
The authors sincerely offer their gratitude to the vice-chancellor for research affairs at the university for his financial support in favor of this research project and all contributors to this study. |
|
| |
References
1. Mugisha JO, Shafer LA, Van der Paal L, Mayanja BN, Eotu H, Hughes P, et al. Anaemia in a rural Ugandan HIV cohort: prevalence at enrolment, incidence, diagnosis and associated factors. Trop Med Int Health 2008 Jun;13(6):788-794.
2. Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. Am J Med 2004 Mar;116(6):420-423.
3. Lijfering WM, Ten Kate MK, Sprenger HG, van der Meer J. Absolute risk of venous and arterial thrombosis in HIV-infected patients and effects of combination antiretroviral therapy. J Thromb Haemost 2006 Sep;4(9):1928-1930.
4. Marcos LA, Majid R, Hamzeh N, Mehta N, Escobar M. An acute intracerebral vein thromboses in AIDS with protein C and S deficiency. Int J STD AIDS 2008 Jan;19(1):59-61.
5. Saber AA, Aboolian A, LaRaja RD, Baron H, Hanna K. HIV/AIDS and the risk of deep vein thrombosis: a study of 45 patients with lower extremity involvement. Am Surg 2001 Jul;67(7):645-647.
6. Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest 1998 May;113(5):1225-1229.
7. Jacobson MC, Dezube BJ, Aboulafia DM. Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis 2004 Oct;39(8):1214-1222.
8. Erbe M, Rickerts V, Bauersachs RM, Lindhoff-Last E. Acquired protein C and protein S deficiency in HIV-infected patients. Clin Appl Thromb Hemost 2003 Oct;9(4):325-331.
9. Bernasconi E, Uhr M, Magenta L, Ranno A, Telenti A; Swiss HIV Cohort Study. Homocysteinaemia in HIV-infected patients treated with highly active antiretroviral therapy. AIDS 2001 May;15(8):1081-1082.
10. Santos JL, Cruz I, Martín Herrero F, Albarrán C, González Matas JM, Martín Luengo C. Recurrent coronary thrombosis, factor V Leiden, primary antiphospholipid syndrome and HIV. Rev Esp Cardiol 2004 Oct;57(10):997-999.
11. Cohen AJ, Philips TM, Kessler CM. Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med 1986 Feb;104(2):175-180.
12. Constans J, Guérin V, Couchouron A, Seigneur M, Ryman A, Blann AD, et al. Autoantibodies directed against phospholipids or human beta 2-glycoprotein I in HIV-seropositive patients: relationship with endothelial activation and antimalonic dialdehyde antibodies. Eur J Clin Invest 1998 Feb;28(2):115-122.
13. Maclean C, Flegg PJ, Kilpatrick DC. Anti-cardiolipin antibodies and HIV infection. Clin Exp Immunol 1990 Aug;81(2):263-266.
14. Sorice M, Griggi T, Arcieri P, Circella A, d’Agostino F, Ranieri M, et al. Protein S and HIV infection. The role of anticardiolipin and anti-protein S antibodies. Thromb Res 1994 Feb;73(3-4):165-175.
15. Guerin J, Feighery C, Sim RB, Jackson J. Antibodies to β2-glycoprotein I–a specific marker for the antiphospholipid syndrome. Clin Exp Immunol 1997 Aug;109(2):304-309.
16. Chretien P, Monier JC, Oksman F, San Marco M, Escande A, Goetz J, et al. Autoantibodies and human immunodeficiency viruses infection: a case-control study. Clin Exp Rheumatol 2003 Mar-Apr;21(2):210-212.
17. Galrão L, Brites C, Atta ML, Atta A, Lima I, Gonzalez F, et al. Antiphospholipid antibodies in HIV-positive patients. Clin Rheumatol 2007 Nov;26(11):1825-1830.
18. Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am 1997 Mar;81(2):449-470.
19. Palomo I, Alarcón M, Sepulveda C, Pereira J, Espinola R, Pierangeli S. Prevalence of antiphospholipid and antiplatelet antibodies in human immunodeficiency virus (HIV)-infected Chilean patients. J Clin Lab Anal 2003;17(6):209-215.
20. Sloand EM, Klein HG, Banks SM, Vareldzis B, Merritt S, Pierce P. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol 1992 Mar;48(3):168-172.
21. Kaslow RA, Phair JP, Friedman HB, Lyter D, Solomon RE, Dudley J, et al. Infection with the human immunodeficiency virus: clinical manifestations and their relationship to immune deficiency. A report from the Multicenter AIDS Cohort Study. Ann Intern Med 1987 Oct;107(4):474-480.
22. Ramezani A, Aghakhani A, Sharif MR, Banifazl M, Eslamifar A, Velayati AA. Anemia Prevalence and Related Factors in HIV-Infected Patients: A Cohort Study. Iranian Journal of Pathology 2008;3(3):125-128.
23. Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Vlahov D; Human Immunodeficiency Virus Epidemiology Research Study Group. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Clin Infect Dis 2002 Jan;34(2):260-266.
24. Levine AM, Berhane K, Masri-Lavine L, Sanchez M, Young M, Augenbraun M, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 2001 Jan;26(1):28-35.
25. Mildvan D, Creagh T, Leitz G; Anemia Prevalence Study Group. Prevalence of anemia and correlation with biomarkers and specific antiretroviral regimens in 9690 human-immunodeficiency-virus-infected patients: findings of the Anemia Prevalence Study. Curr Med Res Opin 2007 Feb;23(2):343-355. |
|