| |
Abstract
Objectives: Cryptococcus neoformans is the most incriminated fungal pathogen causing meningitis in acquired immune deficiency syndrome (AIDS) patients, and is known to constitute a major cause of deaths in AIDS patients. This study thus aimed to determine the baseline sero-prevalence of Cryptococcus neoformans infection in anti-retroviral naïve (ART-naïve) AIDS patients using the serum Cryptococcal antigen (crag) detection method. Baseline effect of variation in CD4 counts, as well as sex and age with sero-positivity for crag were also determined.
Methods: This descriptive cross-sectional study included 150 (61 males and 89 females) ART-naïve AIDS patients attending the Human Immunodeficiency Virus clinic (HIV) at the University of Benin Teaching hospital, Benin City, Nigeria, within the period from February 2011- July 2011. Forty (18 males and 22 females) HIV positive outpatients with CD4 counts >200 cells/µl who were ART-naive were recruited and used as controls. The sero-prevalence of crag in the patients and the control group was measured using the cryptococcal antigen latex agglutination system (CALAS) (Meridian Bioscience, Europe) and CD4 counts were measured using flow cytometry (Partec flow cytometer, Germany).
Results: Of the 150 ART-naïve AIDS patients with CD4 counts £200 cells/µL; 19 (12.7%) were positive for serum Cryptococcal antigen. ART-naïve AIDS patients with CD4 count ≤50 cells/µl had the highest prevalence of serum crag. Lower CD4 counts were significantly associated with positivity for serum crag (p<0.001). Age and sex had no significant effect on the sero-positivity for serum crag. One (2.5%) of the controls was sero-positive for crag. Thus, serum crag was significantly associated with AIDS but not with HIV (p<0.001).
Conclusion: This study uncovers a high prevalence of crag in ART- naïve AIDS patients in Benin City. The prevalence of crag was higher in ART-naïve AIDS patients with lower CD4 counts. There is an urgent need to introduce routine screening for crag in ART- naïve AIDS patients in our locality to reduce the rapid mortality from Cryptococcal meningitis which accounts for a majority of the morbidity factor if undiagnosed during ART therapy.
Keywords: Cryptococcal antigen; ART-naïve AIDS patients; Sero-prevalence; CD4 T cell counts.
Introduction
Cryptococcus neoformans is the most incriminated fungal pathogen causing meningitis in patients with acquired immunodeficiency syndrome (AIDS).1,2 The type of cryptocococcis encountered in human immunodeficiency virus (HIV) is quite different when it has progressed to AIDS. Meningoencephalithis and cryptococcal pneumonia are the common cryptococcal infections found in HIV and AIDS respectively.3 Obligatory and effective administration of anti-retroviral agents in AIDS have been proven to reduce the incidence of cryptocococcis.4 The development of cryptocococcis as with other opportunistic infections during AIDS is associated with a decline in CD4 (Cluster of differentiation) T cell counts in patients.5 Clinical manifestation of infection with Cryptococcus neoformans in AIDS patients is generally more evident at CD4 cells ≤50 cells/µl.6
Cryptocococcis in AIDS is usually asymptomatic and its defining illness is commonly not found in the early course of infection.7 Onset of clinical cryptocococcis in AIDS is found with unspecific clinical symptoms as it is found in most pulmonary and meningeal diseases. Coughing, sweating, fevers, malaise, shortness of breath are common presenting symptoms. The finding of Cryptococcal antigen in the blood represents a condition of systemic invasion with the fungus.8 At this stage there is the capacity of the fungus to disseminate to major parts of the body. The central nervous system is the commonest site of its dissemination, though cutaneous and adrenal dissemination which are rare are also found in some cases.9
The clinical course an ART-naïve AIDS patient that may follow will be rightly tracked and planned if diagnosis of cryptococcal antigenemia is done before initiation of ART. This study thus aimed to determine the prevalence of serum Cryptococcal antigenemia in antiretroviral-naïve AIDS patients with varying CD4 counts at the University of Benin teaching hospital, UBTH, Benin City, Nigeria.
Methods
This descriptive cross-sectional study was carried out in the period of February 2011-July 2011 at the HIV clinic of the University of Benin teaching hospital, Benin City, Nigeria- a referral hospital for AIDS patients in Southern Nigeria. It also houses the South-South regional headquarters of the Institute of human virology, Nigeria, and the Action project molecular research laboratory for HIV diagnosis. Ethical approval for the study was granted by the ethical committee of the University of Benin teaching hospital (UBTH), Benin City, Nigeria. A total of 150 anti-retroviral naïve AIDS patients (61 Males and 89 females) within the age groups <20 to >50 who were counseled, consented for the study and were included in this study. They were patients confirmed to have progressively developed AIDS with CD4 T cell count <200 cells/ µl but had not been on anti-retroviral medications in the period of the study. Forty (18 males and 22 females) HIV patients with CD4 counts >200 cells/µl who were ART-naïve were recruited from the special treatment clinic of UBTH and used as controls. History of cryptococcal meningitis, prior positivity for Cryptococcus neoformans and anti-retroviral use were used as exclusion criteria.
Blood samples were collected by veni-puncture and centrifuged to obtain serum. Laboratory analysis of samples was done at the PEPFAR (President’s Emergency Plan for AIDS Relief) laboratory of UBTH, Benin City. Cryptococcal antigen testing was done using cryptococcal antigen latex agglutination system (CALASR) (Meridian Bioscience; Inc, Europe). This detection kit is simple, sensitive, qualitative and semi - quantitative latex test which detects capsular polysaccharide antigens of Cryptococcus neoformans in Serum and cerebrospinal fluid. Samples were initially pre-treated by incubation at 50°C for 15 mins with CALAS (R) pronase (Meridian Bioscience; Inc, Europe) to reduce non-specific interference with cryptococcal antigen latex test. All tests were carried out according to the manufacturer’s instructions. Determination of CD4 T cell counts in the patients was done using flow cytometry (Partec flow cytometer, Germany). In brief, equal volumes (20 µl) of CD4 PE antibody and Ethylene diamine tetra acetic acid blood was mixed and incubated for 15 mins, and 800 µl of CD4 buffer was added before reading in the cell counter.
Data was analyzed for significance using the chi-square test with Statistical packages for social sciences (SPSS) V. 15 and p<0.05 was taken as significant.
Results
One hundred and fifty patients with immunologic AIDS (CD4 cell count <200 cells/µl) who were not on anti-retroviral therapy were studied. Nineteen (12.7%) were positive for cryptococcal antigen. Prevalence of Cryptococcal antigen was quite varied with CD4 cells levels; CD4 count ≤50 cells/µl had a higher prevalence of cryptococcal antigen (Table 1). This was followed by CD4 cell counts >50-≤100 cells/µl and >100-≤200 cells/µl. Lower CD4 counts were significantly associated with positivity for serum cryptococcal antigen (p<0.001). One (2.5%) of the control was sero-positive for crag. Serum crag was significantly associated with AIDS but not with HIV (p<0.001). Sex was not associated with crag positivity. Also, higher cryptococcal antigenemia were recorded among the age group 31-40 when compared to other age groups (Table 2). However, age and sex had no effect on sero-positivity for crag.
Table 1: Prevalence of serum crag in antiretroviral-naïve AIDS patients in Benin City.
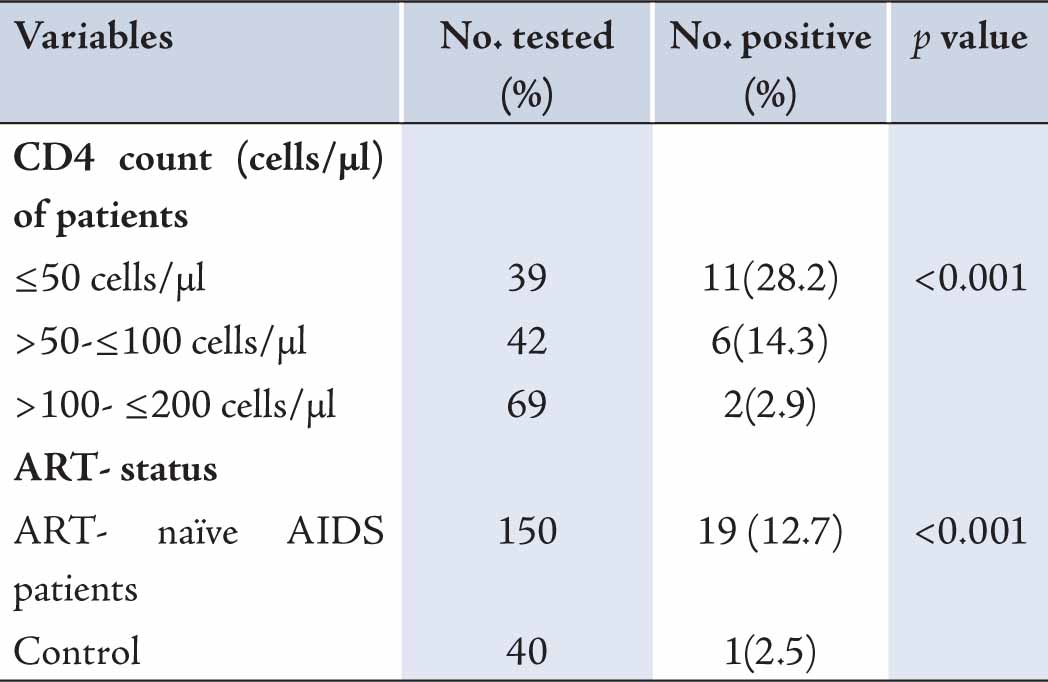
Table 2: Age and sex based prevalence of cryptococcal antigenemia in the studied patients.
Discussion
This study demonstrates a high prevalence of serum cryptococcal antigen in ART-naïve AIDS patients in Benin City, Nigeria. Nineteen (12.7%) of the studied patients were positive for serum cryptococcal antigen. The finding of this study concurs with the 12.2% in Congo, 12.9% in Bangkok and 13.5% in Kampala, Uganda.10-12 The prevalence report of this study is relatively higher than the prevalence rates of other countries; 7% was reported in a retrospective study on ART-naïve AIDS patients in South Africa,13 and 9.2% in Thailand.14 Higher prevalence of cryptococcal antigenemia has been reported in Cambodia (21%).15 The high rate recorded in these patients represents the burden of cryptococcal infection in AIDS patients in Benin City. An earlier study evaluating the prevalence of fungal opportunistic infections in AIDS patients largely on ART-therapy in a hospital using culture and microscopy recovered a 9.7% prevalence of Cryptococcus neoformans.16 The study further goes to confirm the burden of cryptocococcis in AIDS patients at that hospital. Thus, screening for cryptocococcis in AIDS patients should be made a routine.
The distribution of cryptococcal antigenemia was highly varying with CD4 cell levels. Patients with CD4 cell count ≤50 cell/µl had the highest prevalence of serum crag; this was closely followed with patients with CD4 cells >50-≤100 cell/µl and >100-≤200 cells/µl. Several studies evaluating the prevalence of serum cryptococcal antigenemia in AIDS patients have reported a consistently higher prevalence of serum crag in patients with lower CD4 cell counts.17,18 HIV is characteristically associated with T lymphocyte depletion and is highly marked in ART-naïve patients.13,19 Females had more seropositivity for crag when compared to their male counterparts. More men compared to women have been reported to carry a higher burden in the United States,20 but there were no significant difference in the prevalence in men and women in ART-naïve AIDS patients in Benin City. Age also did not play a significant role in serum crag positivity in the patients.
The present study was aimed to determine the baseline serum cryptococcal antigen prevalence in ART-naïve AIDS patients in Benin City, Nigeria. A one year follow-up evaluation of the prevalence of serum crag, mortality and morbidity ratio in ART-naïve AIDS patients who were seropositive or sero-negative for crag in the preliminary survey is being studied and will be reported later. The key to reducing the mortality from Cryptococcal meningitis, which is a consequent of crag positivity, is early diagnosis of crag in ART-naïve AIDS patients prior to initiation of ART therapy and the integration of both ART therapy and primary prophylaxis with Fluconazole; this has been shown to reduce mortality from Cryptococcal meningitis consequent of crag positivity even at CD4 cell count within the range of ≤100 cells/µl to 25 cells/µl.12,21-23
Conclusion
Overall, 12.7% ART-naïve AIDS patients at UBTH, Benin City, were crag positive. Crag prevalence was significantly associated with lower CD4 counts. ART-naïve AIDS patients with CD4 counts ≤50 cells/µl had the highest prevalence. The sero-prevalence of crag was high; thus screening for Cryptococcus neoformans antigen should be made a routine in ART-naïve AIDS patients before initiation of ART therapy to reduce the rapid mortality from Cryptococcal meningitis.
Acknowledgements
We appreciate with thanks the management of University of Benin Teaching Hospital, Benin City for permission to carry out this study. We also thank all technical staff, nurses and midwives of the medical microbiology department, PEPFAR laboratory and HIV clinic departments for their cooperation. All authors participated in laboratory analysis of samples, manuscript preparation and proofreading. No funding was received for the study and no conflict of interest to declare. |
|
| |
References
1. Okongo M, Morgan D, Mayanja B, Ross A, Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 (HIV-1) natural history cohort in Uganda. Int J Epidemiol 1998 Aug;27(4):698-702.
2. Bicanic T, Meintjes G, Rebe K, Williams A, Loyse A, Wood R, et al. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr 2009 Jun;51(2):130-134.
3. van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al; National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med 1997 Jul;337(1):15-21.
4. Michaels SH, Clark R, Kissinger P. Incidence and spectrum of AIDS-defining illnesses among persons treated with antiretroviral drugs. Clin Infect Dis 1999 Aug;29(2):468-469.
5. Benson CA, Kaplan JE, Masor H, Alice P, Holmes KK. Treatment of opportunistic infections among HIV infected Adults and Adolescents. Recommendations and reports. Morbidity and Mortality weekly Report 2004;53:110-112.
6. Pinner RW, Hajjeh RA, Powderly WG. Prospects for preventing cryptocococcis in persons infected with human immunodeficiency virus. Clin Infect Dis 1995;21:103-107 .
7. Cameron ML, Bartlett JA, Gallis HA, Waskin HA. Manifestations of pulmonary cryptococcosis in patients with acquired immunodeficiency syndrome. Rev Infect Dis 1991 Jan-Feb;13(1):64-67.
8. Aberg JA, Watson J, Segal M, Chang LW. Clinical utility of monitoring serum cryptococcal antigen (sCRAG) titers in patients with AIDS-related cryptococcal disease. HIV Clin Trials 2000 Jul-Aug;1(1):1-6.
9. Skiest DJ, Hester LJ, Hardy RD. Cryptococcal immune reconstitution inflammatory syndrome: report of four cases in three patients and review of the literature. J Infect 2005 Dec;51(5):e289-e297.
10. Desmet P, Kayembe KD, De Vroey C. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. AIDS 1989 Feb;3(2):77-78.
11. Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 2007 Aug;12(8):929-935.
12. Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010 Aug;51(4):448-455.
13. Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis 2009 Apr;48(7):856-862.
14. Pongsai P, Atamasirikul K, Sungkanuparph S. The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell counts. J Infect 2010 Jun;60(6):474-477.
15. Micol R, Lortholary O, Sar B, Laureillard D, Ngeth C, Dousset JP, et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr 2007 Aug;45(5):555-559.
16. Aluyi HA, Otajevwo FD, Iweriebor O. Incidence of pulmonary mycoses in patients with acquired immunodeficiency diseases. Niger J Clin Pract 2010;13:78-83.
17. Lara-Peredo O, Cuevas LE, French N, Bailey JW, Smith DH. Cryptococcal infection in an HIV-positive Ugandan population. J Infect 2000 Sep;41(2):195.
18. Kisenge PR, Hawkins AT, Maro VP, McHele JP, Swai NS, Mueller A, et al. Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis. 2007; 10; 7:39.
19. Jarvis JN, Meintjes G, Harrison TS. Outcomes of cryptococcal meningitis in antiretroviral naïve and experienced patients in South Africa. J Infect 2010 Jun;60(6):496-498.
20. Currie BP, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis 1994 Dec;19(6):1029-1033.
21. Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis 2009 Sep;49(6):965-972.
22. Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS 2005 Nov;19(17):2050-2052.
23. Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Centers for Disease Control and Prevention (CDC), National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommend Rep 2009; 58(RR-4):1-207. Quiz CE1-4. |
|