Malaysia is a country located in Southeast Asia and recognized as an upper middle-income country with a population of 31 million. Malaysia’s population comprises many ethnic groups. The Malays predominantly inhabit the Malay Peninsula and parts of Borneo and form the largest community in Malaysia. The second largest ethnic group is the Chinese who make up 23.2% of the population. The Indian community in Malaysia is the smallest of the three main ethnic groups, comprising only 7.0% of the total population. Other minorities include non-Malay indigenous peoples, including ethnic Siamese, Khmers, Chams, and the natives of Sabah and Sarawak. The state of Kelantan is in the Northeast of Peninsular Malaysia, and its people are majority Malay (95.7%).1
The Western Pacific region, of which Malaysia is part of, accounted for nearly 60% of the world’s tuberculosis cases in 2012. Our adjacent neighbors Indonesia, the Philippines, and Thailand were three of the 22 countries with the world’s highest tuberculosis burden.2,3 The World Health Organization (WHO) reported that in 2015, there were one million children and adolescents infected with tuberculosis around the world, and more than 136 000 die annually.2 A recent report from Malaysian Ministry of Health depicted that the proportion of tuberculosis cases among children and adolescents in Malaysia range 17–20% of total tuberculosis cases with increasing trend from 2010 to 2015.4 Meanwhile, the proportion of tuberculosis cases among the pediatric group in the Kelantan state ranges from 1% to 3.01% of total cases from 2000 to 2015 also with an increasing trend.5
The proportion of tuberculosis cases among children and adolescents globally varies from 6.6% to 39.2%.6–12 According to the WHO, the proportion was higher in Africa (16.0% to 39.2%), followed by the Eastern Mediterranean region (35.0%), Southeast Asia (20.0%), the Americas (6.6% to 17.3%), and the Western Pacific region (8.5%).2
The pediatric age group includes all those up to the age of 21.13 Any child or adolescent (10 to 19 years old) infected with Mycobacterium tuberculosis may develop tuberculosis. Most children develop tuberculosis disease within one year of becoming infected. Adolescence is another period during which there is an increased risk of developing active disease.14 Tuberculosis can be classified diagnostically based on anatomical locations and pretreatment acid-fast bacilli (AFB) sputum smear status.15 By anatomical location, tuberculosis can take place in either pulmonary or extrapulmonary sites. Most tuberculosis cases affect the lungs and is known as pulmonary tuberculosis. This form of tuberculosis disrupts the lung parenchyma and leads to structural changes, which are demonstrated by abnormal chest X-ray findings and may be infectious. In addition, tuberculosis can also take place in any anatomical location or as disseminated disease. This form of tuberculosis is known as extrapulmonary tuberculosis and affects organs such as the pleura, lymph nodes, genitourinary tract, abdominal organs, skin, joints and bones, or meninges. Persons with extrapulmonary tuberculosis are usually not infectious unless they sustain pulmonary tuberculosis concomitantly.16
The classification of tuberculosis patients can also be in accordance with the grading of pretreatment sputum smear results on diagnosis. Patients with a chronic cough or suspected with tuberculosis should convey a minimum of two specimens of sputum for microscopic laboratory investigation for the presence of M. tuberculosis. The result will determine whether patients have smear-positive pulmonary tuberculosis or smear-negative pulmonary tuberculosis.17 Pulmonary tuberculosis is the commonest form of tuberculosis in Malaysia; however, extrapulmonary tuberculosis still poses a threat to the nation.15 In children, pulmonary tuberculosis is most common, and sputum smear-negative disease is most frequent. Cases in which sputum cannot be obtained for smear microscopy are considered to be and reported as sputum smear-negative. Extrapulmonary tuberculosis occurs in approximately 20–30% of all cases in children. Adolescents are at particular risk of developing adult-type disease, which is why they are often sputum smear-positive and highly infectious.14
We sought to estimate the total proportion of patients with pediatric tuberculosis, characterize tuberculosis by its anatomical location and sputum smear status, and to determine any association of the sociodemographic and clinical determinants with tuberculosis infection among pediatric patients in Kelantan. The known sociodemographic determinants contributing to tuberculosis infection among children and adolescents are older age,10,18,19 Malay ethnicity,3,20,21 male sex,8,19 low education level,8,9 and residents of rural areas.6 Known clinical determinants associated with tuberculosis infection include cigarette smoking3,8 and positive HIV status.3,19,22 We expect that the findings of our study could provide important criteria of children and adolescents to be prioritized for tuberculosis screening, early diagnosis and prompt treatment, and might mitigate the dynamic transmission of tuberculosis in the community.

Figure 1: Flowchart of the study for factors associated with tuberculosis infection among pediatric patients in Kelantan.
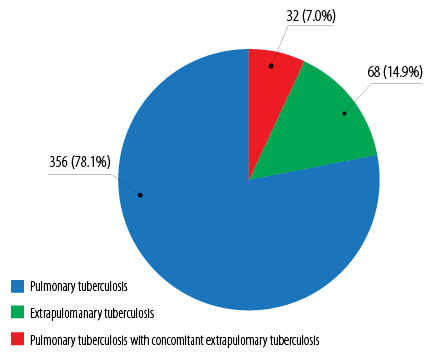
Figure 2: Tuberculosis categories among pediatric tuberculosis patients in Kelantan (n = 456).
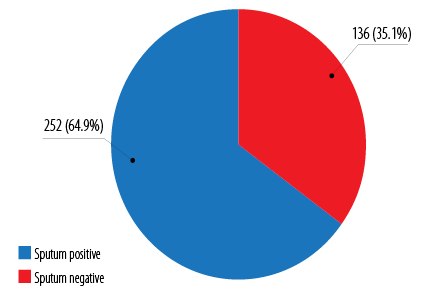
Figure 3: Pulmonary tuberculosis classification based on pretreatment sputum AFB smear status among pediatric tuberculosis patients in Kelantan (n = 388).
Methods
We conducted a comparative cross-sectional study design based on a retrospective record review in the Tuberculosis and Leprosy Control Unit, Kelantan State Health Department.
The reference populations were all pediatric tuberculosis patients in Kelantan, and the study samples were children and adolescents with tuberculosis and non-tuberculosis in Kelantan registered in the Tuberculosis Information System (TBIS) from 2012 to 2015 who fulfilled study inclusion and exclusion criteria. The inclusion criteria were confirmed cases of tuberculosis who were notified to respective District Health Offices in Kelantan and registered in the TBIS from 1 January 2012 to 31 December 2015. Non-tuberculosis cases were tuberculosis contacts with no symptoms, negative Mantoux test, no chest radiograph abnormality, and registered in the TBIS from 1 January 2012 to 31 December 2015. Both cases and non-tuberculosis cases must be of pediatric age (1–19 years old).
The sample size was calculated for each variable of associated factors for tuberculosis disease among pediatric patients using power and sample size calculation software23 as well to compare two independent proportions. The largest estimated sample for each group was 322 using the proportion of non-tuberculosis children and adolescents by the factor of urban residence (0.61),24 an estimated proportion of 0.49, 5% type 1 error, 80% power, and additional 20% missing data.
Table 1: Sociodemographic and clinical characteristics among pediatric respondents registered in Kelantan, 2012–2015 (n = 644).
|
Age, years |
15.9 ± 3.7* |
12.5 ± 5.1* |
|
1–9 |
29 (9.0) |
89 (27.7) |
|
10–19 |
293 (91.0) |
233 (72.4) |
|
Gender |
|
|
|
Male |
145 (45.0) |
166 (51.6) |
|
Female |
177 (55.0) |
156 (48.4) |
|
Ethnicity |
|
|
|
Malay |
292 (90.7) |
315 (97.8) |
|
Others |
30 (9.3) |
7 (2.2) |
|
Education |
|
|
|
Good |
295 (91.6) |
233 (72.4) |
|
Poor |
27 (8.4) |
89 (27.6) |
|
Location |
|
|
|
Non-urban |
256 (79.5) |
219 (68.0) |
|
Urban |
66 (20.5) |
103 (32.0) |
|
Smoking |
|
|
|
No |
275 (85.4) |
302 (93.8) |
|
Yes |
47 (14.6) |
20 (6.2) |
|
HIV status |
|
|
|
Negative |
313 (97.2) |
321 (99.7) |
*Mean±standard deviation.
Data were collected from Kelantan TBIS. TBIS is an online registry set up by the Ministry of Health for surveillance of tuberculosis disease in Malaysia. The retrieved information includes sociodemographic data (age, ethnicity, gender, location of residence, and education level) and clinical factors (HIV and cigarettes smoking status). Using Kelantan TBIS, we found a total of 15 333 tuberculosis cases and tuberculosis contacts among the pediatric group from 2012 to 2015. These were subdivided into tuberculosis cases and non-tuberculosis case sampling frames. From each sampling frame, we used a simple random sampling method to obtain 322 study samples for each group. The flowchart for this study is shown in Figure 1.
We defined good education level as those who attended school at least up to age 10.25 Urban areas were defined as gazette areas with a combined population of 10 000 or more. Meanwhile, rural areas were defined as gazette areas with a combined population of less than 10 000.26
The study was approved by the Human Research and Ethics Committee, Universiti Sains Malaysia (USM/JEPeM/16120592) and the Medical Review and Ethical Committee from National Institute of Health, Ministry of Health, Malaysia (NMRR-16-2348-33521 IIR).
We used SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp) for data entry and analysis. Descriptive statistics with mean and standard deviation (SD), frequency and percentages were calculated. Simple and multiple logistic regression analysis were used to determine factors associated with tuberculosis disease among pediatric patients. All significant variables with a p-value < 0.250 from univariable analysis and clinically important variables were chosen for multiple logistic regression analysis. A p-value < 0.050 was considered statistically significant.
Results
There were 5412 tuberculosis cases, and 36 356 tuberculosis contacts notified and registered in Kelantan TBIS during the four-year study period (2012–2015). The proportion of tuberculosis among pediatric patients in Kelantan was 8.4% (95% confidence interval (CI): 0.08–0.09) or 456 cases out of total 5412 tuberculosis cases. Of these, 356 (78.1%) cases were pulmonary tuberculosis, 68 (14.9%) cases were extrapulmonary tuberculosis, and 32 (7.0%) cases were pulmonary tuberculosis with concomitant extrapulmonary tuberculosis [Figure 2].
The pretreatment sputum AFB smear distribution for all 388 pulmonary tuberculosis (including the pulmonary form with concomitant extrapulmonary tuberculosis) cases was 252 (64.9%) sputum positive cases, and 136 (35.1%) sputum negative cases [Figure 3].
A total of 644 samples were included in this study with 322 samples for each group of tuberculosis and non-tuberculosis cases [Table 1]. The mean age of children and adolescents with tuberculosis was higher compared to those with non-tuberculosis. Children aged 10–19 years old contributed most tuberculosis cases (n = 293, 91.0%). The majority of tuberculosis cases were Malay children and adolescents (n = 292, 90.7%). Females were more prone to contract tuberculosis (n = 177, 55.0%). Tuberculosis cases showed a higher percentage for residents in non-urban areas with 256 cases (79.5%) and among children and adolescents with a good level of education with 295 cases (91.6%). The majority of children and adolescents diagnosed with tuberculosis were non-smokers (n = 275, 85.4%), and had negative HIV status (n = 313, 97.2%).
Multiple logistic regression analysis revealed age, gender, education level, location of residence, and smoking status were the significant associated factors for tuberculosis infection among children and adolescents [Table 2].
Table 2: Factors associated with tuberculosis infection among pediatric patients in Kelantan, 2012–2015, using multiple logistic regression (n = 644).
|
Age |
1.23 (1.17–1.29) |
1.41 (1.29–1.54) |
60.56 (1) |
< 0.001 |
|
Gender |
|
|
12.90 (1) |
< 0.001 |
|
Male |
1.00 |
1.00 |
|
|
|
Female |
1.29 (0.95–1.77) |
1.88 (1.33–2.65) |
|
|
|
Ethnicity |
|
|
13.35 (1) |
< 0.001 |
|
Others |
1.00 |
1.00 |
|
|
|
Malay |
0.22 (0.09–0.50) |
0.17 (0.07–0.44) |
|
|
|
Education |
|
|
39.32 (1) |
< 0.001 |
|
Good |
1.00 |
1.00 |
|
|
|
Poor |
0.24 (0.15–0.38) |
0.20 (0.12–0.33) |
|
|
|
Location |
|
|
11.90 (1) |
0.001 |
|
Urban |
1.00 |
1.00 |
|
|
|
Non-urban |
1.82 (1.28–2.61) |
1.92 (1.33–2.79) |
|
|
|
Smoking |
|
|
16.33 (1) |
< 0.001 |
|
No |
1.00 |
1.00 |
|
|
|
Yes |
2.58 (1.49–4.47) |
3.35 (1.86–6.01) |
|
|
|
HIV infection |
|
|
3.20 (1) |
0.073 |
|
No |
1.00 |
1.00 |
|
|
aSimple logistic regression, bMultiple logistic regression (forward logistic regression method applied).
No multicollinearity and no interaction found. Classification table 74.5% correctly classified.
Area under receiver operating characteristics curve was 79.5%. OR: odds ratio; CI: confidence interval.
Discussion
The proportion of tuberculosis cases among pediatric patients in Kelantan was 8.4% (95% CI: 0.08–0.09). Our finding is similar to the finding from a national data study where children and adolescents comprised about 8.5% out of total tuberculosis cases nationwide.3
We found that the majority of cases among pediatric patients in Kelantan were pulmonary tuberculosis (78.1%). This finding is congruent with a report from the WHO, which showed 70–80% of children with tuberculosis had pulmonary form.14
In Malaysia, a high incidence of tuberculosis was seen among 10 to 19 years old.3 The finding was consistent with our study. Since this age group represents adolescents, it explains the reason for the high incidence of sputum smear-positive tuberculosis in our study because adolescents are at particular risk of developing adult-type disease (that is, they are often sputum smear-positive and highly infectious).14
Our study revealed the mean age for tuberculosis among children and adolescents was 15.9 years old. The finding showed slight discrepancy with a matched case-control study from Brazil that analyzed the risk factors for tuberculosis infection among older children where the mean age of the enrolled patients was 14.4 years.8 Another study on the prevalence of tuberculosis among adolescents in Western Kenya reported the mean age as 14.4 years.6
In our study, age was a significant factor associated with tuberculosis infection (aOR = 1.41, 95% CI: 1.29–1.54; p < 0.001). An increase of one year of age has a 1.4-times increase in the odds of contracting tuberculosis. Similar findings were reported from a cohort study in South Africa among adolescent tuberculosis patients.18 A possible explanation is that older age is associated with engaging in multiple health and disease risk behaviors.27 Moreover, this finding may explain the high incidence of sputum smear-positive tuberculosis, which is believed to be attributed to the older age or the adolescent group. Sputum positive patients are capable of transmitting infection. The number of bacilli depends on the extent of the lesion or the presence of lung cavitation in the pulmonary form of tuberculosis. The larger the cavity or lesion, the larger the amount of bacilli present. Thus, the grading of a positive smear reflects the extent of lesion in a patient as well as being directly proportional to the infectiousness of the case.28
We found a significant association between gender and tuberculosis infection. More than half of patients were female (55.0%), and both female children and adolescents were almost twice as likely to get tuberculosis infection. This finding is in line with three different studies conducted among children and adolescents with tuberculosis in Uganda, Pakistan, and South Africa.9,10,18 All these studies showed that female children and adolescents had higher odds of getting tuberculosis infection. Some studies have shown that males with risk factors such as smoking, alcoholism, drug addiction, and HIV infection acquire tuberculosis more than females, so the sex difference in tuberculosis prevalence was attributable to these factors.29,30 However, there is a possible explanation for the shift of notification rate towards female group. It is possible that female adolescents are simply more likely to use health services during their reproductive years. A study from the US reported that different health-seeking behaviors by females would increase their chance of being diagnosed should they have symptoms of tuberculosis.31 It is known that male patients seek help and use health services less frequently than female patients. Adherence to patriarchal masculine characteristics, such as superiority, independence, self-reliance, and dominance may act as a barrier to men accessing and using health services and consequently prevents diseases from being diagnosed and treated.32
We also found a significant association between other sociodemographic determinants such as ethnicity and education level with tuberculosis infection among children and adolescents. Malay ethnic people comprised the majority of the studied patients most probably due to the high Malay population (95.7%) in Kelantan.1 A conclusion that tuberculosis was prevalent among Malay children and adolescents cannot be made because there were few non-Malay patients in this study. Therefore, the variable cannot be used to test the causal hypothesis.
In contrast with our findings, a study by Liew et al,3 showed that patients without any formal education were 1.94-fold more likely to develop tuberculosis compared to the educated group. Our study revealed that educated children and adolescents were more prone to tuberculosis infection. This finding is supported by a previous study in Brazil, which reported that there is evidence indicating the lack of knowledge and misinformation about tuberculosis among educated group could expose them to the same risk of getting tuberculosis as the uneducated group.33 Possible reasons could be due to lack of tuberculosis awareness or education programs that focus on the educated group. There are many issues that contributed to the lack of tuberculosis awareness or education programs. Among the issues was the community’s reluctance to take ownership of health issues as educated people in some cultures are more reluctant to participate in health activities.34 Alternatively, it may suggest that educated patients have better health-seeking behavior and are well-informed about the need to seek treatment for tuberculosis. This is supported by qualitative research findings on perceptions of tuberculosis and health-seeking behavior in rural Inner Mongolia, China, which reported that less educated people and low-income groups were less likely to seek care, or more likely to seek care at village level where it is cheaper. Both financial and structural barriers were found to stop them from seeking proper health care.35
We also found a significant association between area of residence and tuberculosis infection among children and adolescents. Children and adolescents residing in rural areas had 1.9-times higher odds of getting tuberculosis compared to those in urban areas. The result was in line with a study in Western Kenya, which reported that rural children were more likely to get tuberculosis and accounted for 87% of the total caseload.6 The prevalence of tuberculosis among rural residents are majorly due to lack of knowledge regarding the disease itself. It is reported through a study in a northern rural area of Vietnam that knowledge of causes, transmission routes, symptoms and curability of tuberculosis was low among rural people. They reported that 82% of women and 74% of men residing in rural areas did not know that tuberculosis is caused by bacteria. A large proportion reported that tuberculosis is caused by hard work or it is a hereditary disease.36
The result of this study showed that children and adolescents who smoke cigarettes were three-times more likely to develop tuberculosis infection compared to non-smokers. Findings of this study are in line with another study conducted among older children in Brazil which showed that tobacco smoking has a significant impact on tuberculosis infection.8 In a cross-sectional population survey in South Africa, data on smoking and tuberculin skin test (TST) results of 2401 respondents aged 15 years and above were compared. Out of 1309 current or ex-smokers, 1070 (82%) had a positive TST indicating that cigarette smoking increases the risk of M. tuberculosis infection.37 Another case-control study on the association between tobacco smoke and tuberculosis among children in Thailand revealed that children who were also exposed to tobacco smoke were almost four times more likely to get tuberculosis infection compared to the unexposed group.38 Smokers were more likely to develop tuberculosis due to pathophysiological changes in their respiratory pathway. Smoking not only induces local anatomical disruption, but it also elicits a complex immunological response among smokers.39 A likely explanation is that nicotine turns off the production of TNF-a by the macrophages in the lungs, rendering the patient more susceptible to the development of progressive disease from latent M. tuberculosis infection.40
Multivariable analysis showed no significant association (p = 0.073) between HIV status and tuberculosis infection among children and adolescents, after controlling potential confounding factors. This finding was in agreement with a study in rural Eastern Uganda, which also projected there was no significant association between HIV status and tuberculosis infection among children and adolescents.19 However, it is well-known from previous studies that tuberculosis is among the commonest opportunistic infection in HIV-infected patients.41 HIV-positive people are 20–30 times more likely than HIV-negative people to develop tuberculosis in countries with a generalized HIV epidemic. Tuberculosis is also a major cause of morbidity in HIV-infected children. HIV-infected children have a 20–25-fold higher incidence of contracting tuberculosis than uninfected children, with an overall tuberculosis incidence in South African HIV-infected children of 9.2% (95% CI: 0.14–0.97).42
Among limitations of the study are its small sample size of HIV-positive patients and limited samples from other ethnicities besides Malay. Moreover, the use of a cross-sectional study design may only reveal the association between sociodemographic and clinical factors with tuberculosis rather than a proper sequence of cause and effect.
Conclusion
The most common of tuberculosis disease among pediatric patients in Kelantan was the pulmonary form and sputum smear-positive. The identified risk factors for tuberculosis were older age, female gender, good education level, rural residence, and cigarette smoking. Our study widens the comprehension and understanding of the epidemiology of tuberculosis among pediatric patients and its associated factors. Future cohort studies including a wider range of other factors such as nutritional status, social factors (specifically household condition), and molecular level factors contributing to tuberculosis disease are needed. It is hoped that the findings from this study may assist health authorities to design a better and comprehensive plan for national tuberculosis control, focusing more attention towards the pediatric age group.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors would like to thank the Director General of Health Malaysia for allowing us to perform data collection from TBIS database for tuberculosis. Ethical approval was obtained from Ministry of Health. Our gratitude also goes to the Kelantan Tuberculosis and Leprosy Control Unit for their assistance during data collection.
references
- 1. Department of Statistics. Population and housing census of Malaysia: Population distribution and basic demographic characteristics 2010. Malaysia: Department of Statistics; 2010. p. 24. [cited 12 January 2017]. Available from: https://www.mycensus.gov.my/banci/www/index.php?&id=3&page_id=40&filename=penerbitan&aid=53.
- 2. World Health Organization. Global Tuberculosis Report 2016. Switzerland: World Health Organization; 2016. p. 5-30. [cited 12 January 2017]. Available from: http://apps.who.int/medicinedocs/documents/s23098en/s23098en.pdf.
- 3. Liew SM, Khoo EM, Ho BK, Lee YK, Mimi O, Fazlina MY, et al. Tuberculosis in Malaysia: predictors of treatment outcomes in a national registry. Int J Tuberc Lung Dis 2015 Jul;19(7):764-771.
- 4. Annual Tuberculosis Report. Malaysia: Ministry of Health; 2016.
- 5. Kelantan State Health Department. Kelantan Annual Tuberculosis Report. Malaysia: Kelantan State Health Department; 2016.
- 6. Nduba V, Hoog AH, Mitchell E, Onyango P, Laserson K, Borgdorff M. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. Int J Infect Dis 2015 Jun;35:11-17.
- 7. Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis 2004 May;8(5):636-647.
- 8. Stevens H, Ximenes RA, Dantas OM, Rodrigues LC. Risk factors for tuberculosis in older children and adolescents: a matched case-control study in Recife, Brazil. Emerg Themes Epidemiol 2014 Dec;11(1):20.
- 9. Waako J, Verver S, Wajja A, Ssengooba W, Joloba ML, Colebunders R, et al. Burden of tuberculosis disease among adolescents in a rural cohort in Eastern Uganda. BMC Infect Dis 2013 Jul;13(1):349.
- 10. Zafar M. Prevelence of latent tuberculosis and associated risk factors in children under 5 years of age in Karachi, Pakistan. The Journal of Association of Chest Physicians 2014;2(1):16-24.
- 11. Uppada DR, Selvam S, Jesuraj N, Lau EL, Doherty TM, Grewal HM, et al; TB Trials Study Group. Incidence of tuberculosis among school-going adolescents in South India. BMC Public Health 2016 Jul;16(1):641.
- 12. Wood R, Liang H, Wu H, Middelkoop K, Oni T, Rangaka MX, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 2010 Apr;14(4):406-412.
- 13. Simon GR, Baker CN, Barden GA, Brown OW, Hachell JM, Hardin AP, et al. Recommendations for preventive pediatric health care. Committee on Practice and Ambulatory Medicine, 2013-2014. Pediatrics 2015 Sep;136(3). [cited 12 January 2017]. Available from: https://www. http://pediatrics.aappublications.org/content/136/3/e727.
- 14. World Health Organization. Roadmap for childhood tuberculosis: towards zero deaths. Geneva, Switzerland: World Health Organization; 2013.
- 15. Clinical practice guidelines: management of tuberculosis. 3rd ed. Ministry of Health: Malaysia; 2012.
- 16. Core curriculum on tuberculosis: what the clinician should know. 4th ed. Division of Tuberculosis Elimination: Centers for Disease Control Prevention; 2000.
- 17. World Health Organization. Treatment of tuberculosis guidelines. 4th ed. Geneva, Switzerland: World Health Organization; 2010.
- 18. Mahomed H, Ehrlich R, Hawkridge T, Hatherill M, Geiter L, Kafaar F, et al. TB incidence in an adolescent cohort in South Africa. PLoS One 2013;8(3):e59652.
- 19. Mumpe-Mwanja D, Verver S, Yeka A, Etwom A, Waako J, Ssengooba W, et al. Prevalence and risk factors of latent Tuberculosis among adolescents in rural Eastern Uganda. Afr Health Sci 2015 Sep;15(3):851-860.
- 20. Hooi LN. Case-finding for pulmonary tuberculosis in Penang. Med J Malaysia 1994 Sep;49(3):223-230.
- 21. Nissapatorn V, Kuppusamy I, Rohela M, Anuar AK, Fong MY. Extrapulmonary tuberculosis in Peninsular Malaysia: retrospective study of 195 cases. Southeast Asian J Trop Med Public Health 2004;35(Suppl 2):39-45.
- 22. Moyo S, Verver S, Mahomed H, Hawkridge A, Kibel M, Hatherill M, et al. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int J Tuberc Lung Dis 2010 Feb;14(2):149-154.
- 23. Dupont WD, Plummer WD: ‘Power and Sample Size Calculations: A Review and Computer Program’, Controlled Clinical Trials 1990; 11:116-28.
- 24. van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child 1999 May;80(5):433-437.
- 25. Malaysia education for all: Mid-decade Assessment Report 2000-2007. Malaysia: Ministry of Education; 2008. p. 18-20.
- 26. IPH. National Health and Morbidity Survey 2015 (NHMS 2015). Vol I: Methodology and general findings. Malaysia: Institute for Public Health 2015.
- 27. DuRant RH, Smith JA, Kreiter SR, Krowchuk DP. The relationship between early age of onset of initial substance use and engaging in multiple health risk behaviors among young adolescents. Arch Pediatr Adolesc Med 1999 Mar;153(3):286-291.
- 28. Rajpal S, Dhingra VK, Aggarwal JK. Sputum grading as predictor of treatment outcome in pulmonary tuberculosis. Indian J Tuberc 2002;49(3):139-142.
- 29. Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis-a systematic review. BMC Public Health 2008 Aug;8:289.
- 30. Silveira JM, Sassi RA, de Oliveira Netto IC, Hetzel JL. Prevalence of and factors related to tuberculosis in seropositive human immunodeficiency virus patients at a reference center for treatment of human immunodeficiency virus in the southern region of the state of Rio Grande do Sul, Brazil. J Bras Pneumol 2006 Jan-Feb;32(1):48-55.
- 31. Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis 1998 Feb;2(2):96-104.
- 32. Smith JA, Braunack-Mayer A, Wittert G. What do we know about men’s help-seeking and health service use? Med J Aust 2006 Jan;184(2):81-83.
- 33. Mussi TV, Traldi MC, Talarico JN. Knowledge as a factor in vulnerability to tuberculosis among nursing students and professionals. Rev Esc Enferm USP 2012 Jun;46(3):696-703.
- 34. Country Health Plan. 10th Malaysia Plan 2011-2015. Malaysia: Ministry of Health; 2011. p. 26-27.
- 35. Zhang T, Liu X, Bromley H, Tang S. Perceptions of tuberculosis and health seeking behaviour in rural Inner Mongolia, China. Health Policy 2007 May;81(2-3):155-165.
- 36. Hoa NP, Thorson AE, Long NH, Diwan VK. Knowledge of tuberculosis and associated health-seeking behaviour among rural Vietnamese adults with a cough for at least three weeks. Scandinavian Journal of Public Health 2003;31(62_suppl):59-65.
- 37. den Boon S, van Lill SW, Borgdorff MW, Verver S, Bateman ED, Lombard CJ, et al. Association between smoking and tuberculosis infection: a population survey in a high tuberculosis incidence area. Thorax 2005 Jul;60(7):555-557.
- 38. Charoenca N, Kungskulniti N, Tipayamongkholgul M, Sujirarat D, Lohchindarat S, Mock J, et al. Determining the burden of secondhand smoke exposure on the respiratory health of Thai children. Tob Induc Dis 2013 Mar;11(1):7.
- 39. Abal AT, Jayakrishnan B, Parwer S, El Shamy A, Abahussain E, Sharma PN. Effect of cigarette smoking on sputum smear conversion in adults with active pulmonary tuberculosis. Respir Med 2005 Apr;99(4):415-420.
- 40. Davies PD, Yew WW, Ganguly D, Davidow AL, Reichman LB, Dheda K, et al. Smoking and tuberculosis: the epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg 2006 Apr;100(4):291-298.
- 41. Ghate M, Deshpande S, Tripathy S, Nene M, Gedam P, Godbole S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis 2009 Jan;13(1):e1-e8.
- 42. Naidoo K, Naidoo K, Padayatchi N, Abdool Karim Q. HIV-associated tuberculosis. Clinical and Developmental Immunology 2010;2011:585919.