|
Abstract
Nerve sheath myxoma is a rare benign tumor of the peripheral nerves. It typically presents as a painless, firm, and slow growing nodule with a predilection for extremities mostly fingers and knees. Microscopically, it has characteristic multilobules of spindle cells in an abundant myxoid stroma. The cells are strongly positive for S-100 protein. However, this rare tumor is usually misdiagnosed as other more common benign neuronal tumors. This report describes a rare case of nerve sheath myxoma involving the palmar surface of a 23-year-old female. Clinically, it was diagnosed as a fibroma. It was excised and the final diagnosis was made after histopathological and comprehensive immunohistochemical examination of the specimen. The clinicopathological features of this rare tumor and its important differential diagnoses are discussed along with a brief review of the literature.
Keywords: Nerve sheath myxoma; Palmar nodule.
Introduction
Nerve sheath myxoma is a rare and benign tumor which usually arises from the cutaneous peripheral nerves. It affects the extremities of middle age adults and has an uncertain pathogenesis; however, some authors suggest a close relationship with schwannoma. The typical morphology of NSM is that of spindle cells with abundant myxoid stroma. The cells are strongly S-100 protein positive. We present a case of a morphologically typical nerve sheath myxoma which occurred in the palm of a 23- year-old female.
Case Report
A 23-year-old female presented with a painless and itchy nodule over the thenar eminence of her left palm. Clinical history showed that she had this nodule for seven years without any change in size. The patient's past medical history was unremarkable and her clinical laboratory profile was reported as being within normal limits. On examination, the lesion was a 0.5 cm, skin-colored nodule, firm, mobile and tender. It was completely excised and sent for histopathological assessment with a clinical diagnosis of fibroma.
Histopathological Findings
Gross examination showed a relatively well demarcated nodule occupying the dermis and subcutaneous tissue. Microscopic examination revealed a multilobulated mass with a relatively well defined fibrous border located in the dermis and part of subcutaneous tissue. The lobules were of variable sizes and separated by thin fibrous septae. They were composed of relatively uniform spindle cells embedded in abundant myxoid stroma (Fig. 1A). No mitoses were seen. Alcian blue special stain highlighted the presence of abundant mucin in the background (Fig. 2A). Immunohistochemical (IHC) study showed that the tumor spindle cells were positive for S-100 protein (Fig. 2B) and vimentin. They were, however, negative for GFAP, desmin, neurofilament protein, and CD34.
Discussion
Nerve sheath myxoma (NSM) was first described by Harkin and Reed in 1969 as a benign tumor originating in the peripheral nervous system.1 Since that time, the lesion was reported under various names including neurothekeoma,2-4 myxomatous perineuroma,2 myxoma of the nerve sheath,1,2 and dermal nerve sheath myxoma.5 This reflects the uncertain histogenesis of this tumor. However, most of the cases reported in the literature showed strong positivity for S-100 protein favoring a Schwann cell origin.6
NSM is common in middle aged adults with a male:female ratio ranging from 1:1 to 1:2.1,6 NSM is most commonly located on the fingers, knees, or peritibial region. Other locations affected are hips, thighs, lower leg/calf, ankle, foot, trunk, head and neck, and palmar surfaces of the hands.6 Rare cases were reported in mucosal locations and in the CNS.7-9 Our case was in the palm of a 23-year-old female. NSM typically presents as a firm, slow-growing, painless nodule with size ranging from 0.4 to 4.5 cm. In our case, the nodule was painless but tender. Most NSM specimens consisted of a single piece of tissue that is sometimes covered by skin. On cut section, small, well-demarcated, translucent or whitish mucoid nodules are often noted. Microscopically, the lesion can involve either the dermis only or the dermis and subcutis. Very rarely the lesion is located solely in the subcutis and adjacent superficial skeletal muscles. It is characterized by multilobular structures and each lobule is composed of spindle, stellate-shaped, and sometimes, epitheloid cells arranged in swirling, lamellar and often concentric patterns. These cells are embedded in an abundant myxoid stroma. Mild nuclear pleomorphism and rare mitoses may be present.6
Immunohistochemically, tumor cells are strongly positive for S-100 protein, GFAP and vimentin. EMA-positive perineural cells are present in small numbers usually best seen at the borders of the myxoid nodules.6 In our case, S-100 protein and Vimentine were positive but GFAP and CD34, and neurofilament protein were negative. Electron microscopic findings in one study showed that NSM has three types of cells. Type 1 cell matches the ultrastructral features of the perineural cells; type 3 cell has features similar to the Schwann cells. These two types are characterized by the presence of basement membrane and may in fact be the functional variants of the same sheath cells. Type 2 cell lacks the basement membrane which qualifies it as an endoneurial fibroblast.10
The behavior of NSM is benign with higher propensity for local recurrences.4,6 In one study, the recurrence rate was 47%.6 NSM has been regarded as a subtype of neuothekeoma (so-called myxoid variant of neurothekoma).11 However, some authors suggest that NSM is a distinct clinicopathologic entity that is unrelated to neurothekeoma.6 In a study of 178 cases of neuothekoma, all tumors were negative for S-100 protein and GFAP. An origin from fibroblasic cells with the ability to differentiate into myofibroblasts was hence postulated.12
Nerve sheath myxoma can be distinguished from neurothekeoma by its immunohistochemical and histopathological features (Table 1).13 Other possible differential diagnoses include neurofibroma, schwannoma, and perineuroma. Cellular and mixed type neurothekeoma have much less myxoid matrix, more spindling and sometimes plumper epithelioid cells, more nuclear variability and greater mitotic activity than true nerve sheath myxoma. They are S-100 protein negative.6
Schwannoma typically shows nuclear palisading with proliferation of spindle shaped cells (Antoni type A). In myxoid neurofibroma, the myxoid matrix is observed only in a limited area and is mixed with bundles of collagen. Furthermore, neurofibroma often contains a number of mast cells and neurites having positive reactivity of neurofilament protein.14 NSM as in our case has atypical multilobular architecture and the myxoid matrix is found in all areas without bundles of collagen. Neurofilament protein is negative.
Table 1: Histopathological and immunohistochemical differential diagnoses of nerve sheath myxoma.
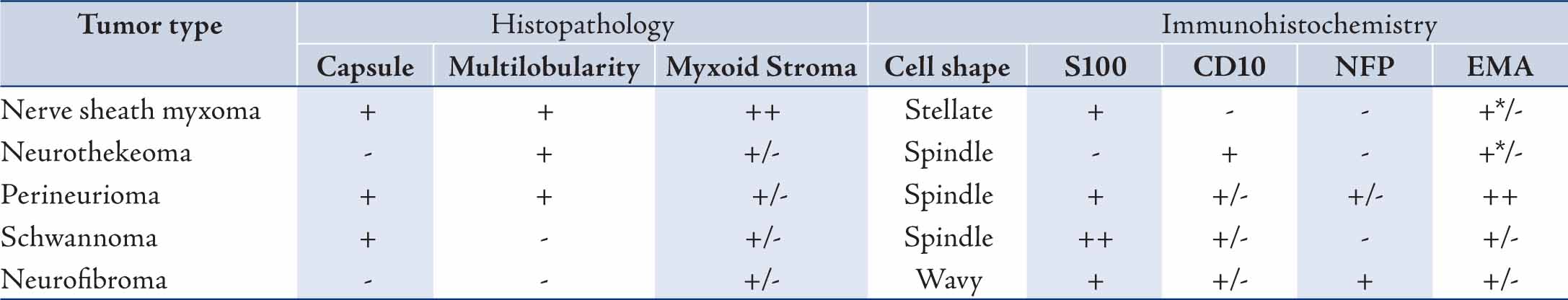
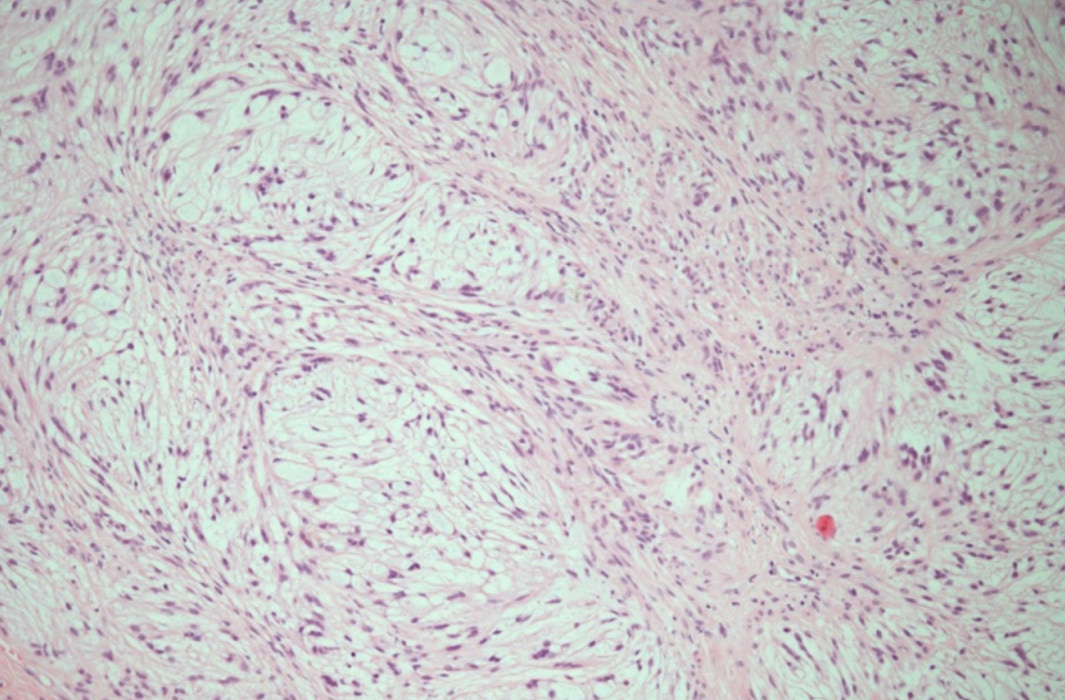
Figure 1A: Nerve sheath myxoma: Note the presence of vague spindle cells nodules (arrow head) in a myxoid background. (hematoxylin and eosin stain × 100).
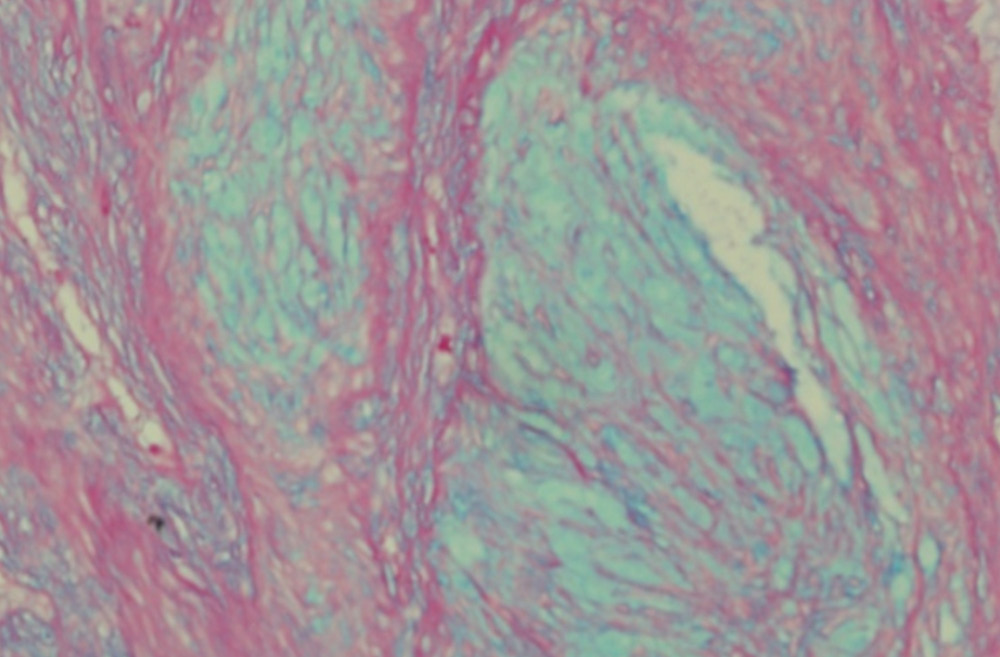
Figure 2A: Nerve sheath myxoma: Strong positive blue staining of the myxoid stroma with the Alcian Blue (pH 2.5) mucin stain, (Alcian blue × 200).
Figure 2B: Nerve sheath myxoma: Spindle cells showing strong positive nuclear and cytoplasmic staining with S-100 protein stain. (IHC stain × 200).
Conclusion
In patients presenting with painless palmar nodule the possibility of palmar nerve sheath myxoma should be considered and investigated. The lesion should be differentiated from the myxoid variant of neurothekeoma and other mimicking spindle cell lesions by both morphological and immunohistochemical studies.
Acknowledgements
I would like to thank Dr. Ammar Al Rikabi for his kind motivation and guidance while writing this paper. |