Actinomycosis is a granulomatous suppurative disease that is caused by non-spore forming, anaerobic, branching, gram-positive bacteria belonging to the genus Actinomyces.1,2 Actinomyces are part of the normal oral, intestinal, and genital tracts flora; hence, they require tissue injury or disruption of the mucosal barrier to invade adjacent structures.3 Although cervicofacial, thoracic, and abdominopelvic forms are the most common presentations, rare manifestations such as hepatic, cardiac, and breast involvements have been reported in the literature. Disseminated actinomycosis is clinically indistinguishable from metastatic malignancies or more common chronic infections. Thus, many physicians called it the great mimicker.4,5 Here, we report a case of disseminated actinomycosis in a 59-year-old female who presented with nonspecific abdominal pain and weight loss. We aim to highlight the importance of considering actinomycosis as a possible cause of abdominal pain, particularly in the presence of constitutional symptoms.
Case report
A 59-year-old female known to have sickle cell trait presented to our tertiary care center with a three-month history of abdominal pain and weight loss. The abdominal pain was central, non-radiating, moderately severe, progressive, and colicky with no aggravating or relieving factors. It was associated with unintentional weight loss (10% of the patient’s weight), fatigue, and anorexia for the same period.
She had no other symptoms, such as fever, night sweats, vomiting, or changes in urinary or bowel habits. She underwent uncomplicated total abdominal hysterectomy for uterine leiomyomatosis years before the presentation. There was no history of smoking or alcohol misuse.
Table 1: Initial laboratory investigations.
|
White blood count |
20.6 × 109/L (3.6–9.6 × 109/L) |
|
Hemoglobin |
9 g/dL (12–14.5 g/dL) |
|
Mean corpuscular volume |
74.3 fL (80–97 fL) |
|
Platelets count |
656 × 109/L (150–400 × 109/L) |
|
Creatinine |
37 μmol/L (44–97 μmol/L) |
|
Urea |
3.5 mmol/L (3.2–8.2 mmol/L) |
|
Erythrocyte sedimentation rate |
121 mm/hour (< 20 mm/hour) |
|
C-reactive protein |
162 mg/L (0–3 mg/L) |
|
Total protein |
86 g/L (64–82 g/L) |
|
Albumin |
27 g/L (35–52 g/L) |
|
Globulin |
59 g/L (15–30 g/L) |
|
Alkaline phosphatase |
186 U/L (50–136 U/L) |
|
G-glutamyl transferase |
141 U/L (5–55 U/L) |
|
Alanine aminotransferase |
19 U/L (< 33 U/L) |
On examination, she was afebrile, vitally stable, pale, and had mild diffuse abdominal tenderness with no palpable lymphadenopathy. She showed no abnormal neurological, cardiac, or respiratory signs.
Initial diagnostic workup confirmed the presence of microcytic hypochromic anemia, leukocytosis, mildly elevated liver enzymes, and significantly raised inflammatory biomarkers [Table 1].
Abdominal ultrasound revealed multiple hypoechoic lesions in the liver. Further evaluation by abdominal computed tomography (CT) scan confirmed the presence of a large heterogeneous irregular hepatic mass with intrahepatic biliary duct dilatation. Additionally, it revealed a circumferential wall thickening of the transverse colon and stomach [Figure 1]. Chest X-ray revealed right lower zone infiltrate with obliteration of right costophrenic angle, which was further evaluated by CT scan that confirmed the presence of right pleural effusion accompanied by right lower lung infiltrate. It also revealed moderate pericardial effusion [Figure 2].

Figure 1: Contrast-enhanced CT scan of the abdomen in the late arterial phase shows heterogeneously enhancing lobulated lesion in the right hepatic lobe (red arrows).
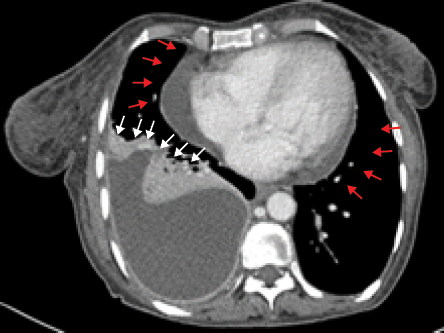
Figure 2: Contrast-enhanced CT scan of the chest shows a large encysted right pleural effusion
(white arrows) and moderate pericardial effusion (red arrows).
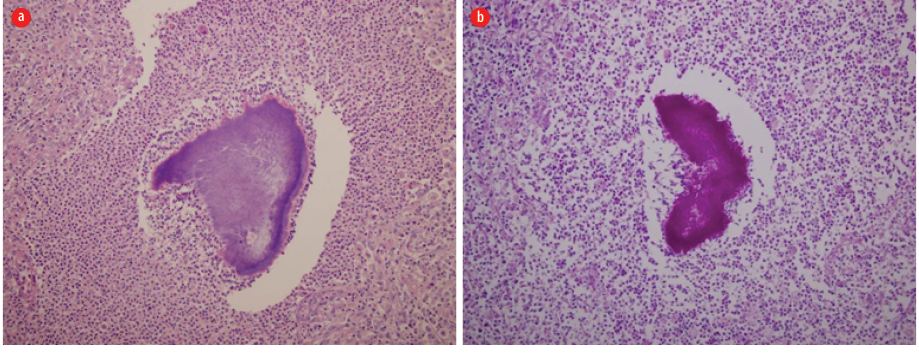
Figure 3: (a) An Actinomyce surrounded by extensive neutrophils, macrophages, lymphocytes, and plasma cells (hematoxylin and eosin stain, magnification = 20 ×). (b) Periodic acid-Schiff stain (magnification = 20 ×).
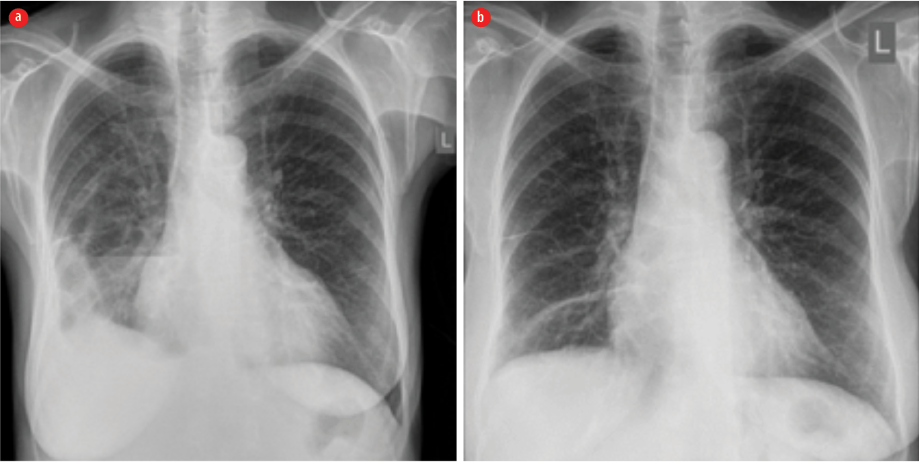
Figure 4: (a) Chest X-ray with a large right pleural effusion. (b) Complete resolution.
The initial impression was a malignant process with hepatic, pleural, and pericardial involvements. Endoscopy of the upper and lower gastrointestinal tract was performed and reported normal. A bone scan to assess the presence of bone metastasis was negative. The patient ultimately had exploratory laparotomy and a liver biopsy, which showed an extensive inflammation with lymphoplasmacytic infiltrates, macrophages, abscesses, and Actinomyces (positive periodic acid-Schiff stain) [Figure 3].
The patient was diagnosed as a case of disseminated actinomycosis involving the liver, heart, intestine, and lungs. Treatment was commenced with high-dose ampicillin, after which the patient showed considerable improvement in clinical symptoms and a drop in inflammatory markers. However, by the second week, her fever spiked again. We conducted septic workup considering the possibility of hospital-acquired sepsis. The repeated chest X-ray revealed worsening of the right-sided pleural effusion [Figure 4] that mandated thoracentesis and insertion of a chest drain.
The patient showed significant clinical improvement with the chest tube drainage. By the third week, the drainage was removed with no further recollection of the fluid, but she started to have a major allergic reaction to ampicillin in the form of a generalized urticarial reaction. Hence, the antibiotic was changed to doxycycline, which she tolerated well, and she was fit for discharge by the end of the fourth week with a plan for the continuation of doxycycline for a total of four to six months based on the clinical response. A follow-up CT scan, after two months, showed substantial interval reduction in pleural effusion, pericardial effusion, and liver abscess. Interestingly, a repeated scan by the end of the sixth month revealed complete radiological resolution with significant clinical improvement in her appetite and weight (she gained 10 kg over six months).
Discussion
Actinomycosis is a rare infection worldwide with an incidence of less than one person in every 300 000 per year owing to the dramatic improvement of personal hygiene, availability of the antibiotics, and meticulous aseptic surgical techniques.6 To the best of our knowledge, three cases of isolated forms have been reported previously in Bahrain, but none have such a disseminated picture.7,8
Reported case series revealed that males are more likely to be infected than females with a male-to-female ratio of 3:1.3,4 Risk factors include dental procedures, poor dental hygiene, abdominopelvic surgeries, and immunocompromised states. However, actinomycosis has been reported even in the absence of such factors.
Cervicofacial actinomycosis, the commonest form, presents in up to 60% of cases typically occurs post-surgical manipulation of the oral cavity.9,10 Approximately, 20% of cases have thoracic manifestations that involve the lung parenchyma, pleura, and chest wall.11 Furthermore, less than 20% of cases have abdominopelvic involvement.12
Of note, the diagnosis of actinomycosis can be challenging due to the absence of specific clinical signs and symptoms.13 In addition, the pathogenesis of actinomycosis mandates a breach in the mucosal barrier, subsequent suppurative granulomatous inflammatory response, extensive fibrosis crossing the underlying tissue planes, and invasion of the surrounding organs. This process mimics malignancy in its pathological, clinical, and radiological presentation and such fibrosis may impede the identification of characteristic of sulfur granules.14–15 Moreover, Actinomyces require up to 21 days for culture and isolation in a clinical microbiology laboratory, which obviously might delay the diagnosis, particularly if empirical treatment with antibiotics was initiated before obtaining the biopsy and thus might result in masking the underlying causative organism.
Antibiotics remain the cornerstone in the treatment of actinomycosis regardless of the form, severity, and extent of disease,3,16 whereas surgical options are reserved for complicated cases or in refractory cases to conservative management.17 The surgical approach, without concurrent antibiotic therapy, is rarely successful as a definitive treatment.18 The prognosis of the disease remains excellent, particularly if diagnosed and treated in the early stages.12
Conclusion
Even though it is a rare infection, a high index of suspicion is required to diagnose actinomycosis. Early diagnosis improves the prognosis and prevents morbidity, including unwarranted surgical interventions. When uncertainty of the diagnosis persists, a biopsy should be considered to identify the definitive causative agents.
Disclosure
The authors declared no conflict of interests.
Acknowledgements
We would like to thank Dr Nawal Al Hamar for providing us with the radiological images.
references
- 1. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev 2015 Apr;28(2):419-442.
- 2. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ 2011;343:d6099.
- 3. Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, Breton P, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 2014 Jul;7:183-197.
- 4. Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope 1984 Sep;94(9):1198-1217.
- 5. Ngow HA, Wan Khairina WM. Cutaneous actinomycosis: the great mimicker. J Clin Pathol 2009 Aug;62(8):766.
- 6. Jin SL, Lee HP, Kim JI, Chin JY, Choi SJ, Joo M, et al. A case of endobronchial actinomycosis. Korean J Intern Med 2000 Dec;15(3):240-244.
- 7. Al-Sindi K, El-Shenawi H, Al-Hilli f. Actinomycosis of the gallbladder. Bahrain Medical Bull 2006;28:1-7.
- 8. Farid IA, Malik AK. Primary actinomycosis presenting as chronic perianal abscess report of a case and review of literature. Bahrain Med Bull 1995;17(3).
- 9. Oostman O, Smego RA. Cervicofacial actinomycosis: diagnosis and management. Curr Infect Dis Rep 2005 May;7(3):170-174.
- 10. Volante M, Contucci AM, Fantoni M, Ricci R, Galli J. Cervicofacial actinomycosis: still a difficult differential diagnosis. Acta Otorhinolaryngol Ital 2005 Apr;25(2):116-119.
- 11. Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J 2003 Mar;21(3):545-551.
- 12. Heo SH, Shin SS, Kim JW, Lim HS, Seon HJ, Jung SI, et al. Imaging of actinomycosis in various organs: a comprehensive review. Radiographics 2014 Jan-Feb;34(1):19-33.
- 13. Yamamoto W, Kono H, Maekawa M, Fukui T. The relationship between abdominal pain regions and specific diseases: an epidemiologic approach to clinical practice. J Epidemiol 1997 Mar;7(1):27-32.
- 14. Peabody JW Jr, Seabury JH. Actinomycosis and nocardiosis. A review of basic differences in therapy. Am J Med 1960 Jan;28:99-115.
- 15.Sharkawy AA, Chow AW. Cervicofacial actinomycosis. [Cited 2018 December 20]. Available from: https://www.uptodate.com/contents/cervicofacial-actinomycosis.
- 16. Moniruddin A, Begum H, Nahar K. Actinomycosis: an update. Med Today 2010;22(1):43-47.
- 17. Song JU, Park HY, Jeon K, Um SW, Kwon OJ, Koh WJ. Treatment of thoracic actinomycosis: a retrospective analysis of 40 patients. Ann Thorac Med 2010 Apr;5(2):80-85.
- 18. Robati RM, Niknezhad N, Bidari-Zerehpoush F, Niknezhad N. Primary cutaneous actinomycosis along with the surgical scar on the hand. Case Rep Infect Dis 2016;2016:5943932.