|
Abstract
Objective: This study assesses the prevalence of hypoglycemia among patients presenting at the University of Benin Teaching Hospital, Benin City, Nigeria with cholestasis of infancy.
Methods: During a period of five years, forty patients aged between 15 days and 12 months who presented with cholestasis of infancy, were admitted and screened for hypoglycemia, using Accutrend glucometer. For patients with low blood glucose values, blood samples were further analyzed, using the standard glucose-oxidase method.
Results: Of the 2,835 patients admitted over a five-year period, 40 (1.4%) had cholestasis of infancy, giving an incidence of 14 cases per 1000 admissions, with a sex ratio of 2.1: 1 in favour of males. Nine (22.5%) of the 40 infants with cholestasis had at least one blood glucose concentration less than 2.6 mmol/L (hypoglycemia). Of the nine hypoglycemic infants, three (33.3%) had one blood glucose concentration less than 1.6 mmol/L (severe hypoglycemia). Seven (77.8%) of the nine hypoglycemic infants were diagnosed in the first 36 hours of admission. Lethargy and poor feeding were observed in three infants with severe hypoglycemia and three of them died. Six (66.7%) of the hypoglycemic infants were below 3 months of age.
Conclusions: Hypoglycemia was observed among patients with cholestasis of infancy and the prevalence was higher among infants below 3 months of age.
Keywords: Hypoglycemia; Cholestasis; Infancy; Neonatal cholestasis syndrome.
Introduction
Among infants, hypoglycemia is a common metabolic problem encountered in association with a variety of disorders, such as malaria, kwashiorkor, and hepatic diseases (e.g., hepatitis, cirrhosis, metabolic liver disorders).1-4 The coexistence of hypoglycemia and other diseases may influence the outcome or clinical course of the primary disease, if not appropriately addressed.
The term cholestasis refers to a group of disorders associated with bilirubin excretion and accompanied by a rise in serum conjugated bilirubin levels and often, bile salts and phospholipids.3 Some of the key clinical features of cholestasis of infancy are jaundice persisting beyond the age of 14 days, jaundice with elevated serum conjugated bilirubin fraction (>2.0 mg/dl or >20% of total bilirubin), variably acholic stool, dark urine that stain the diaper yellow, bilirubinuria, and hepatomegaly.4,5 Clinically, the hallmark of cholestasis is itching but this may not be recognized in early infancy. Itching becomes apparent after the age of six months.5 Before the age of six months, irritability is a common feature of itching. The estimated incidence of cholestasis of infancy is 1 in 2,500 infants.1,6
The liver plays an important role in the maintenance of blood glucose levels, which is highly regulated by insulin and insulin counterregulating hormones. Thus, a patient with liver dysfunction manifesting with cholestasis of infancy may be at increased risk of developing hypoglycemia.7-9 In infants, hypoglycemia is defined as blood glucose concentration of 2.5 mmol/L and below.10
The association of cholestasis of infancy with hypoglycemia has been documented.5,11 Some studies have demonstrated links between hepatic dysfunction, hypoglycemia and congenital hypopituitarism.12,13 Although the mechanisms are not clearly understood, involvement of growth hormone and cortisol have been hypothesized.11,13,14 Lablanc et al.11 indicated that cortisol deficiency might be the main endocrine abnormality responsible for both hypoglycemia and liver dysfunction. Reports of other studies support this view.14-16 Thus, adrenal function tests should be requested for in infants with hypoglycemia and liver dysfunction.11,14-16 On the other hand, other studies have linked hypoglycemia and liver dysfunction (manifesting as cholestasis of infancy) to mitochondrial respiratory enzyme defect.17,18 Although the biochemical mechanisms of occurrence of hypoglycemia in association with cholestasis of infancy have been studied,11-18 the prevalence of hypoglycemia among patients with cholestasis of infancy has not been well documented, especially in Nigeria.
This study assesses the prevalence of hypoglycemia among patients presenting with cholestasis of infancy at the University of Benin Teaching Hospital, Nigeria.
Methods
All infants (aged 15 days to 12 months) diagnosed with cholestasis of infancy and admitted between January 2004 and December 2008 into the Under-Five-Pediatric Ward of the Department of Child Health, University of Benin Teaching Hospital (UBTH) were recruited into the study after obtaining informed consent from the parents/caregivers. In this study, the presence of all the standard clinical features were used for the diagnosis of cholestasis of infancy.4,5
The glucometer (Acutrend meter product 128485) was used for measurement of blood glucose.19 Laboratory confirmation of blood glucose level was requested for all infants with blood glucose level below 3.0 mmol/L. Appropriate monitoring of blood glucose level was observed for all the infants. For infants with at least one blood glucose level below 2.0 mmol/L, the glucose level was monitored for at least one day after the level had returned to normal or after the therapy had been discontinued. When the blood glucose level was below 1.6 mmol/L or the infant was symptomatic (irrespective of blood glucose value), intravenous administration of 10.0% glucose was started immediately.7,10 Hematocrit values of all the infants were determined and recorded and blood films were prepared and examined for malaria parasite and liver function tests (LFT) were performed using standard procedures.19 All the infants were examined clinically for features of kwashiorkor and congenital conditions.
In the present study, infants with blood glucose level of 2.5 mmol/L and below were characterized as hypoglycemic.10 Appropriate statistical methods were used for analysis of data; odd ratios were computed and Z-test was used in ascertaining the significance of differences in proportions with p-value set at <0.05.
Results
During the 5-year period covered by this study, a total of 2,835 children were admitted into the Under-Five Ward in UBTH; 40 (1.4%) of them had cholestasis of infancy, giving an incidence of 14 cases per 1000 admissions. Of the 40 infants with cholestasis of infancy, 27 (67.5%) were males and 13 (32.5%) were females, giving a male-to-female ratio of 2.1:1. Nine (22.5%) of the 40 infants with cholestasis had at least one blood glucose level below 2.6 mmol/L (hypoglycaemia). Of the nine hypoglycemic infants, three (33.3%) had one blood glucose level below 1.6 mmol/L (severe hypoglycemia). The remaining 6 (66.7%) had one or more blood glucose concentration between 1.6 and 2.5 mmol/L. The three infants with severe hypoglycemia died. Seven (77.8%) of the hypoglycemic infants were diagnosed within the first 36 hours of admission while two (22.2%) were diagnosed between 36 to 72 hours of admission. Lethargy and poor feeding were present in the three infants with severe hypoglycemia. Hypoglycemia was observed only among infants who were between 15 days and 6 months old. (Table 1)
Table 1: Age at presentation and prevalence of hypoglycemia among patients with cholestasis of infancy.
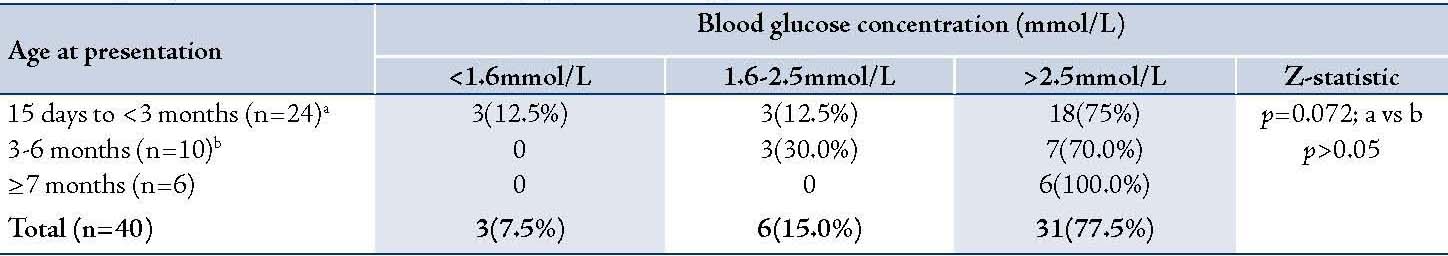
Table 2: Gender distribution among the 40 infants with cholestasis of infancy.
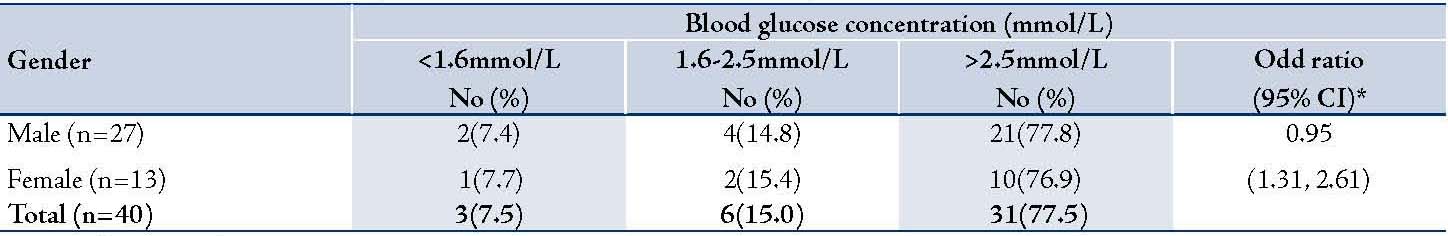
Table 3: Distribution of serum alkaline phosphatase (ALP) levels among the 40 patients with cholestasis of infancy
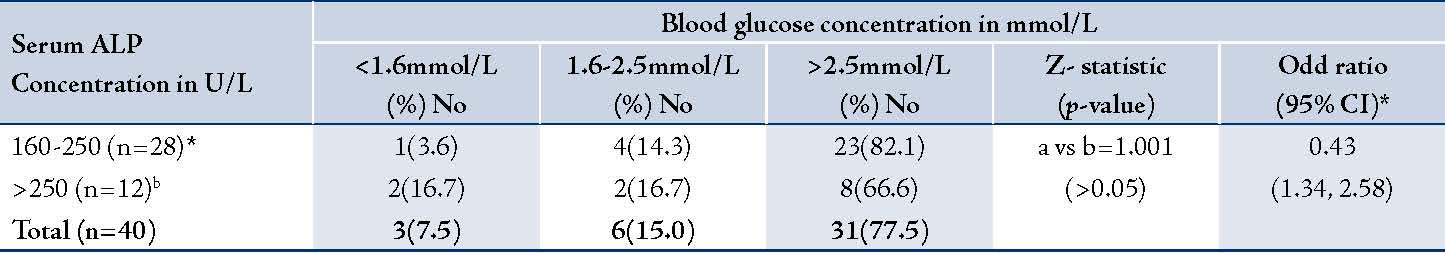
Table 4: Distribution of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels among the 40 patients with cholestasis of infancy.
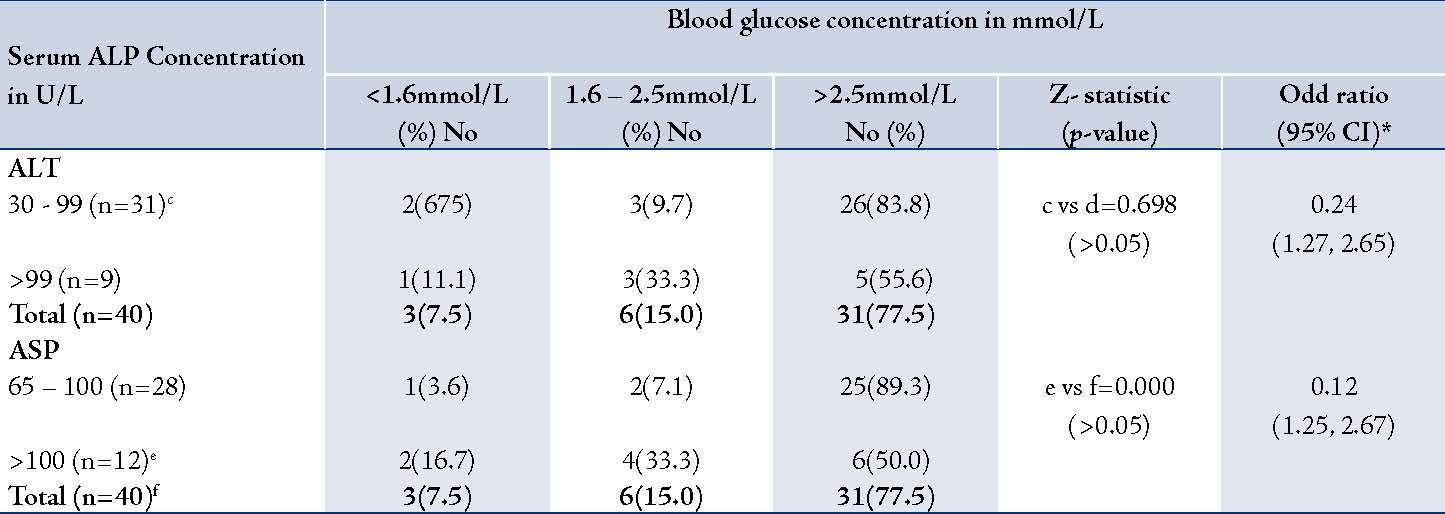
None of the infants older than 6 months at the time of presentation had hypoglycemia, (Table 1). The prevalence of hypoglycemia was 22.2% in males and 23.1% in females, (Table2); p>0.05. There was no association between the serum alkaline phosphatase level and the occurrence of hypoglycemia (Table 3). Similarly, there was no association between the serum aminotransferase levels and occurrence of hypoglycemia, (Table 4). Clinical examination revealed normal external male genitalia and bilateral scrotal testis. The female infants also had normal female external genitalia. There was no pedal edema. Total serum bilirubin concentrations ranged from 6.5-17.6 mg/dl with the conjugated fraction ranging from 1.5-5.4 mg/dl and generally was more than 20% of the total serum bilirubin concentrations. Serum albumin concentration ranged from 36-48 g/L. The blood glucose values obtained using the Accutrend glucometer correlated well with the values obtained from the hospital central laboratory.
Discussion
The overall prevalence (22.5%) of hypoglycemia among patients with cholestasis of infancy was 3.7 times lower than that reported by Leblanc et al. in France.11 The lower prevalence observed in the present study may be accounted for by differences in study population. Their study population was patients with either primary or secondary cortisol deficiency.11
Although infants with cholestasis whose age was between 15 days and 3 months had a higher frequency of occurrence of hypoglycemia than the other age groups, the difference was not statistically significant. This in keeping with the report of Lablanc et al.11 in which they observed that of six patients with hypoglycemia associated with cholestasis of infancy, four were below three months of age. In the present study, although there were more males than females with cholestasis of infancy, the prevalence of hypoglycemia was similar, suggesting that there was no gender difference in the frequency of occurrence of hypoglycemia. Lablanc et al.11 indicated that of the five infants with cholestasis, four (80.0%) were males. In contrast, the Gonc et al.16 reported two cases, one male (3 months of age)and one female (6 months of age), with both infants manifesting episodes of hypoglycemia. These authors stated that cholestasis among their patients was due to primary or secondary cortisol deficiency.11,16 It has been suggested that the age of appearance of the cortisol deficiency is an important predictor of occurrence of cholestatic hepatitis, and consequently, occurrence of hypoglycemia.15,16,20 It is assumed that cortisol deficiency manifesting in the neonatal or early infancy period causes cholestatic hepatitis. For instance, five of six patients with isolated cortisol deficiency who presented beyond early infancy did not have cholestatic hepatitis.20 Such an assumption cannot be made from the present study because it was not designed to identify the specific etiology of cholestasis of infancy.
In the present study, the serum levels of alkaline phosphatase and aminotransferases did not appear to influence the prevalence hypoglycemia. The reason is not clear. Whether this is related to the fact that hepatocyte mass in some metabolic disorders is lost by apoptosis rather than cell necrosis, resulting in serum aminotransferase being only moderately elevated1 and consequently, unrelated to occurrence of hypoglycemia is not clear.
Although the present study had some limitations, the report may act as an introduction to a more comprehensive prospective study to examine the etiology of hypoglycemia associated with cholestasis of infancy and assess its link with the status of the adrenal glands as well as the outcome.
Conclusion
Overall, hypoglycemia was observed among patients with cholestasis of infancy especially in the first three months of life.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Suchy FJ. Neonatal cholestasis. Pediatr Rev 2004 Nov;25(11):388-396.
2. Solomon T, Felix JM, Samuel M, Dengo GA, Saldanha RA, Schapira A, et al. Hypoglycaemia in paediatric admissions in Mozambique. Lancet 1994 Jan;343(8890):149-150.
3. Cherry C. Hyperbilirubinaemia. In: Gomella TC, ed. Neonatology: Management, Procedures, On-call problems, Diseases, and Drugs. 5th ed. New York, 2004:381-395.
4. Ling SC. Congenital cholestatic syndromes: what happens when children grow up? Can J Gastroenterol 2007 Nov;21(11):743-751.
5. Thapa BR. Neonatal cholestatic syndrome. In: Parthasarathy A, ed. IAP Textbook of Pediatrics. 4th edition, New Delhi, 2009: 682-692.
6. Dick MC, Mowat AP. Hepatitis syndrome in infancy–an epidemiological survey with 10 year follow up. Arch Dis Child 1985 Jun;60(6):512-516.
7. Williams AF. Hypoglycaemia of the newborn: a review. Bull World Health Organ 1997;75(3):261-290.
8. Bender DA, Mayes PA. Gluconeogenesis and the control of blood glucose. In: Murray RK, Granner DK, Rodwell VW eds. Harper’s Illustrated Biochemistry, 27th edition, New York, MacGraw Hill Companies Inc, 2006: 167-176.
9. De Bruyne R, Van Biervliet S, Vande Velde S, Van Winckel M. Clinical practice: neonatal cholestasis. Eur J Pediatr 2011 Mar;170(3):279-284.
10. Sperling MA. Hypoglycaemia. In: Kleigman RM, Behrman RE, Jenson HB, Stanton BF. Nelson Textbook of Pediatrics, 18th ed, Philadelphia, Saunders Elsevier: 2007: 655-669.
11. Leblanc A, Odièvre M, Hadchouel M, Gendrel D, Chaussain JL, Rappaport R. Neonatal cholestasis and hypoglycemia: possible role of cortisol deficiency. J Pediatr 1981 Oct;99(4):577-580.
12. Spray CH, Mckiernan P, Waldron KE, Shaw N, Kirk J, Kelly DA. Investigation and outcome of neonatal hepatitis in infants with hypopituitarism. Acta Paediatr 2000 Aug;89(8):951-954.
13. Choo-Kang LR, Sun CC, Counts DR. Cholestasis and hypoglycemia: manifestations of congenital anterior hypopituitarism. J Clin Endocrinol Metab 1996 Aug;81(8):2786-2789.
14. BerberoGlu M. YiGlt S. O’Cal G, Kansu A, Tarcan A, Girgin N, Suskan E. Isolated deficiency of glucocorticoids presenting with cholestasis. Pediatr Int 1998;40(4):378-380.
15. Lacy DE, Nathavitharana KA, Tarlow MJ. Neonatal hepatitis and congenital insensitivity to adrenocorticotropin (ACTH). J Pediatr Gastroenterol Nutr 1993 Nov;17(4):438-440.
16. Gönç EN, Kandemir N, Andiran N, Ozön A, Yordam N. Cholestatic hepatitis as a result of severe cortisol deficiency in early infancy: report of two cases and review of literature. Turk J Pediatr 2006 Oct-Dec;48(4):376-379.
17. Goncalves I, Hermans D, Chretien D, Rustin P, Munnich A, Saudubray JM, et al. Mitochondrial respiratory chain defect: a new etiology for neonatal cholestasis and early liver insufficiency. J Hepatol 1995 Sep;23(3):290-294.
18. Mochel F, Slama A, Touati G, Desguerre I, Giurgea I, Rabier D, et al. Respiratory chain defects may present only with hypoglycemia. J Clin Endocrinol Metab 2005 Jun;90(6):3780-3785.
19. Cheesbrough M. District Laboratory Practice in Tropical Countries (Part 1). Cambridge, Cambridge University Press, 2006: 340-348.
20. Yordam N, Kandermir N. Familial glucocorticoid deficiency: clinical spectrum and endocrine details in five Turkish children (Abstract). Horm Res 1996;46(Suppl):92.
|