Malignant mesenchymoma (MM) is a rare malignant soft tissue neoplasm that usually contains two or more different mesenchymal components. This term was first used by Stout in 1948.1 Only a few cases of MM have been described in the literature to date and to the best of our knowledge, none in the salivary gland. Other mesenchymal tumors with two or more different morphologies (e.g, dedifferentiated liposarcoma, dedifferentiated chondrosarcoma and liposarcoma with cartilaginous, smooth muscle, and osseous differentiation) should be excluded for the correct diagnosis.2 Immunohistochemistry (IHC) plays a major role in the accurate diagnosis of this entity. These tumors are usually high grade and seen in different organs.3
We report a rare case of MM in the submandibular salivary gland of an elderly patient.
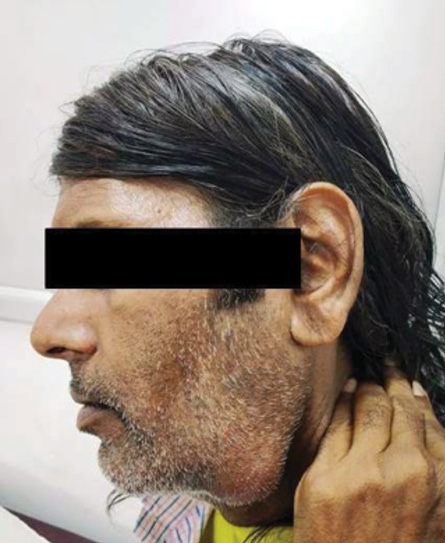
Figure 1: Swelling under left jaw 7 × 4 cm in size.

Figure 2: Fine-needle aspiration cytology showing spindle cell clusters with prominent nuclear enlargement with atypia and myxoid stroma (Giemsa, magnification = 400 ×).

Figure 3: Cut surface of submandibular salivary gland showing homogenous greyish white appearance without necrosis.
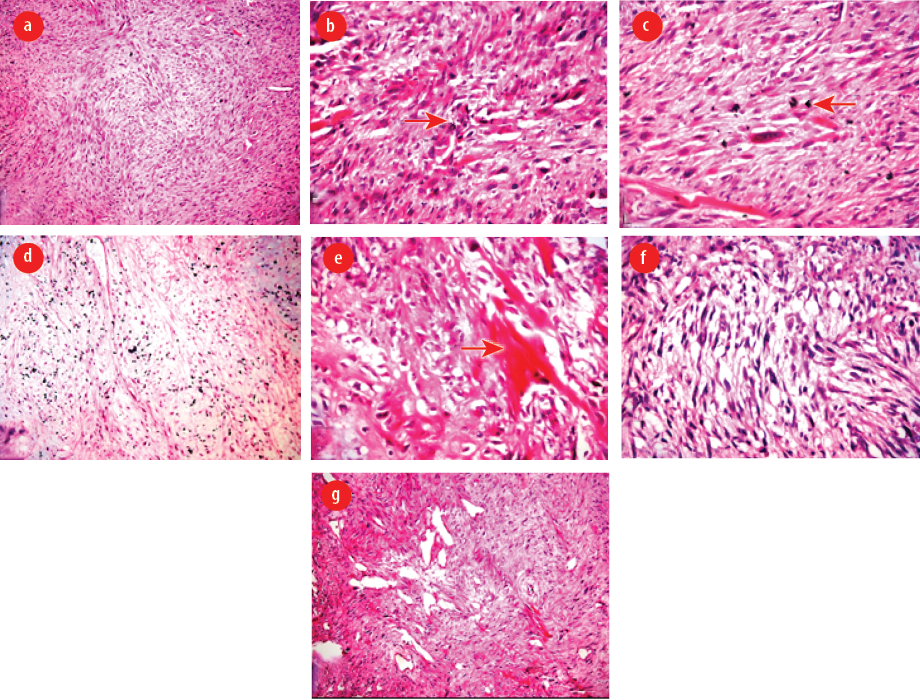
Figure 4: (a) Predominant spindle cell sarcoma (hematoxylin and eosin (H&E), magnification = 100 ×), (b) marked cellular polymorphism with typical and atypical mitotic figures (H&E, magnification = 400 ×), (c) spindle-shaped tumor cells with abundant eosinophilic cytoplasm (H&E, magnification = 400 ×), (d) areas of chondrosarcoma (H&E, magnification = 100 ×), (e) areas of osteosarcoma with osteoid production (H&E, magnification = 400 ×), (f) areas of leiomyosarcoma (H&E, magnification = 400 ×), and (g) areas of rhabdoid differentiation (H&E, magnification = 100 ×).

Figure 5: (a) Focal positivity for desmin (magnification = 400 ×). (b) Positivity for smooth muscle actin (magnification = 400 ×). (c) S-100 positivity in chondrosarcomatous region (magnification = 400 ×). (d) Strong positivity for p53 (magnification = 400 ×). (e) Strong positivity for Ki-67 (magnification = 400 ×).
(f) Negativity for pan-cytokeratin (magnification = 400 ×).
Case Report
A 54-year-old man presented to the outpatient department of Otorhinolaryngology with swelling under his left jaw, increasing in size for the last one month. It was initially about 0.5 × 0.5 cm but gradually increased to the present size of 7 × 4 cm [Figure 1]. The patient had smoked tobacco for 30 years. There was no associated fever, pain, voice change, breathing difficulty, dysphagia, weight loss, and/or history of tuberculosis exposure.
Local examination revealed a left-sided neck swelling of 6.5 × 4 cm extending from the lower border of the mandible to the submandibular area. The overlying skin was tense, but the local temperature was not raised. There was no redness or ulceration. The swelling was immobile and firm. General physical and ear, nose, throat examination revealed no abnormalities. Ultrasonography of the neck showed evidence of a hypoechoic, irregular, lobulated lesion 4.8 × 4.1 × 3.3 cm in size in the left submandibular gland. There was also evidence of multiple hypoechoic lesions of average size, 1.3 × 0.5 cm. The rest of the structures in the neck were normal. Based on the ultrasonography findings, we suspected a malignancy and histopathological confirmation was advised. We could not do further preoperative evaluation in the form of magnetic resonance imaging as the patient did not give consent due to financial constraints.
Fine-needle aspiration cytology (FNAC) was performed using a 26G needle and 10 mL syringe. Smears were stained with Papanicolaou and Giemsa. Smears showed the presence of tightly clustered pleomorphic spindle cells. The spindle cell clusters showed nucleomegaly along with atypia with the presence of myxoid stroma [Figure 2].
On FNAC findings, the lesion was suspected as malignant salivary gland neoplasm with two differential diagnoses of carcinoma ex pleomorphic carcinoma or malignant myoepithelioma, and histopathological confirmation was advised.
Intraoperatively, the marginal mandibular nerve was stretched. During surgical excision of the tumor, blunt dissection was performed, taking care not to damage the marginal mandibular nerve and lingual nerve. The whole tumor mass was excised and sent for histopathology. On gross examination, the mass measured 5.5 × 4 × 3 cm in size. The external surface was grey and white, and firm in consistency. The cut surface showed a homogenous greyish white appearance without necrosis [Figure 3]. Histological examination revealed the presence of a malignant spindle cell component [Figure 4 a]. The tumor cells were spindle in shape with eosinophilic cytoplasm. There was a proliferation of poorly differentiated immature mesenchymal cells with hyperchromatic nuclei with marked atypia. The mitotic figures were higher than 5 mitoses per 50 high power fields. There was marked cellular polymorphism along with numerous typical and atypical mitotic figures [Figure 4 b and c]. There were also areas of chondrosarcoma having atypia with an abundant cartilaginous matrix with hyperchromatic nuclei [Figure 4 d]. The section also showed areas of osteosarcoma showing pleomorphic spindle cells producing eosinophilic osteoid [Figure 4 e]. There was also a component of leiomyosarcoma containing hypercellular spindle cells with nuclear atypia and abnormal mitotic figures [Figure 4 f]. There was rhabdoid differentiation at places showing moderately cellular areas with sheets of small spindle cells having deeply eosinophilic cytoplasm with elongated tails (tadpole cells) and inconspicuous nucleoli [Figure 4 g]. The spindle cells showed focal positivity to desmin and smooth muscle actin (SMA) [Figure 5 a and b]. S-100 was positive in chondrosarcomatous region [Figure 5 c]. p53 and Ki-67 were strongly positive throughout [Figure 5 d and e]. The tumor was negative for pan-cytokeratin [Figure 5 f]. Based on morphology and IHC, a diagnosis of MM was made. Postoperatively, after receiving radiotherapy, the patient was disease-free at three, six, 12, 18, and 24 months follow-up.
Discussion
MM is a unique tumor that consists of two or more different malignant mesenchymal components like osteosarcoma, chondrosarcoma, leiomyosarcoma, and liposarcoma. It can occur at all locations in the body, including the retroperitoneum, soft tissue of lower limbs, heart, pleura, liver, orbit, larynx, thyroid, bone, mediastinum, and urinary bladder.3 The occurrence of this tumor in the submandibular salivary gland is unusual and, to the best of our knowledge, has not been reported to date in the English literature. It appears to arise from primitive mesenchymal cells with the capacity for totipotent differentiation, but the histogenesis remains uncertain.3 While diagnosing MM, we have to consider that some sarcomas have osteoid and chondroid differentiation that may imitate this entity.3
On cytology, the diagnosis was given as a malignant salivary gland neoplasm with possibilities of carcinoma ex pleomorphic or malignant myoepithelioma. But on histology, there was the presence of four distinct malignant mesenchymal components. The tumor cells were negative for pan-cytokeratin, which excluded the epithelial component. Partial positivity for SMA and desmin showed myoblastic differentiation. The chondrosarcomatous region was neoplastic and showed positivity for S-100. The differential diagnoses can be spindle cell sarcoma with osseous and cartilaginous regions and malignant peripheral nerve sheath tumors (MPNST) wherein cartilaginous and bone formation is common. But in MPNST, the osseous and cartilaginous component is reactive, while in our case, it was malignant. S-100 was negative in spindle cell component, being positive only in the chondrosarcomatous region, ruling out MPNST. The other tumors that mimic MM with two or more components include liposarcoma with smooth muscle component; cartilaginous or osseous differentiation, dedifferentiated liposarcoma, and dedifferentiated chondrosarcoma. All these entities can be differentiated from MM in that the myoblastic component in MM is not dedifferentiated, and the rest of the components are neoplastic.
As the tumor consisted of different components despite a predominant homogenous macroscopic finding on the cut surface, it is important to study all areas of a tumor using IHC for correct histological diagnosis of MM.
The prognosis of MM is still controversial. According to some authors, it is a high-grade malignant neoplasm with a poor survival rate.4,5 Brady et al,4 studied cases of MM and found two- and three-year survival rates as 75% and 37% in femoral and retroperitoneal cases, respectively. Sharma et al,5 reported that all four patients of MM died of disease within two years. In the present case, the patient was uneventful at three, six, 12, 18, and 24 months follow-up. The presence of different components may suggest outcomes as reported by few authors. A case of MM in a 40-year-old patient reported that the presence of a rhabdomyosarcomatous component corresponds to a poor prognosis. He also suggested no significant difference in prognosis with respect to tumor size, site, and gender.6
There is insufficient data to suggest the best treatment option or modality to cure the condition as the tumor is rare.7–9 Only chemotherapy or radiotherapy were found to be ineffective. Thus, a multidisciplinary approach including surgery, chemotherapy, and radiotherapy may be useful for such tumors as in our case.9
Conclusion
We reported a case of MM containing undifferentiated spindle cell sarcoma, leiomyosarcoma, chondrosarcoma, osteosarcoma, and areas with rhabdoid differentiation in a 54-year-old male arising from the submandibular salivary gland. As it is a rare tumor and probably the first case in the salivary gland, more literature is needed to decide the management and prognosis of this
rare tumor.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Stout AP. Mesenchymoma, the mixed tumor of mesenchymal derivatives. Ann Surg 1948 Feb;127(2):278-290.
- 2. Kudawara I, Araki N, Nakanishi H, Mano M, Ishiguro S. Malignant mesenchymoma of the lower leg. J Clin Pathol 2001 Nov;54(11):877-879.
- 3. Li YF, Yu CP, Wu ST, Dai MS, Lee HS. Malignant mesenchymal tumor with leiomyosarcoma, rhabdomyosarcoma, chondrosarcoma, and osteosarcoma differentiation: case report and literature review. Diagn Pathol 2011 Apr;6:35.
- 4. Brady MS, Perino G, Tallini G, Russo P, Woodruff JM. Malignant mesenchymoma. Cancer 1996 Feb;77(3):467-473.
- 5. Sharma TC, Huvos AG, Grabstald H. Retroperitoneal malignant mesenchymoma. J Urol 1971 Jul;106(1):60-66.
- 6. Adachi T, Oda Y, Sakamoto A, Terashi T, Tamiya S, Hachitanda Y, et al. Prognostic factors in the so-called malignant mesenchymoma: a clinicopathological and immunohistochemical analysis. Oncol Rep 2003 Jul-Aug;10(4):803-811.
- 7. Newman KD, Schisgall R, Reaman G, Guzzetta PC. Malignant mesenchymoma of the liver in children. J Pediatr Surg 1989 Aug;24(8):781-783.
- 8. Hauser H, Beham A, Schmid C, Uranüs S. Malignant mesenchymoma: a very rare tumor of the peritoneum. Case report with a review of the literature. Langenbecks Arch Chir 1991;376(1):38-41.
- 9. Tanimura S, Saito Y, Honma K, Koizumi K. Surgical case of giant malignant mesenchymoma in the posterior mediastinum that recurred in the bilateral mediastinum. J Nippon Med Sch 2008 Aug;75(4):212-215.