|
Abstract
Keratins are a diverse group of structural proteins that form thei ntermediate filament network responsible for maintaining the structural integrity of keratinocytes. In humans, there are around 30 keratin families divided into two groups, namely, acidic and basic keratins, which are arranged in pairs. They are expressed in a highly specific pattern related to the epithelial type and stage of cellular differentiation. A total of 54 functional genes exist which codes for these keratin families. The expression of specific keratin genes is regulated by the differentiation of epithelial cells within the stratifying squamous epithelium. Mutations in most of these genes are now associated with specific tissue fragility disorders which may manifest both in skin and mucosa depending on the expression pattern. The keratins and keratin-associated proteins are useful as differentiation markers because their expression is both region specific and differentiation specific. Antibodies to keratin are considered as important tissue differentiation markers and therefore are an integral aid in diagnostic pathology. The present review discusses the structure of keratin, the various types of keratin and their distribution and the disorders associated with keratinization with special emphasis on the disorders of the oralcavity. A brief note on the clinical significance of keratin is also mentioned.
Keywords: Keratinization; Keratins; Distribution; Keratinization disorders; Keratin antibodies.
Introduction
Epithelia function to protect the underlying tissues from environmental influences such as physical damage, infection, dessication, UV radiation, heat loss, and to maintain homeostasis.1 Oral epithelium is classified into three types based on their morphology and specific pattern of differentiation: keratinized stratified squamous epithelium (masticatory mucosa distributed in hard palate and gingiva), non-keratinized stratified squamous epithelium (buccal mucosa, labial mucosa) and specialized mucosa (dorsal surface of the tongue).2 An important aspect of stratified squamous epithelia is that the cells undergo a terminal differentiation program that results in the formation of a mechanically resistant and toughened surface composed of cornified cells that are filled with keratin filaments and lack nuclei and cytoplasmic organelles. In these squames, the cell membrane is replaced by a proteinaceous cornified envelope that is covalently cross linked to the keratin filaments, providing a highly insoluble yet flexible structure that protects the underlying epithelial cells.1
Keratinization, also termed as cornification, is a process of cytodifferentiation which the keratinocytes undergo when proceeding from their post germinative state (stratum basale) to finally differentiated, hardened cell filled with protein, constituting a structurally and functionally distinct keratin containing surface layer such as stratum corneum.3 Most of the eukaryotic cell is composed of cytoskeleton which is made of three components classified on the basis of their diameter and physicochemical properties into microfilaments, intermediate filaments, and microtubules. Microfilaments are the smallest filaments of cytoskeleton with a diameter of 7 nm while microtubules are the largest filamentous structures with a diameter of about 20 nm.4
Intermediate filaments, which serve as a scaffold for the cytoskeleton, are chemically very stable, long and unbranched filaments that aggregate into bundles of varying diameter ranging from 7 to 12 nm. Keratins that form the intermediate filaments are expressed exclusively in the epithelial cells regardless of the germ layer origin of these cells.4 Among the various families and sub-families of intermediate filament proteins, keratin is an important type due to its high molecular diversity. Keratins play a major functional role in the integrity and mechanical stability of both the single epithelial cells and via cell to cell contacts of that of the epithelial tissues.5 There are around 30 families of keratin proteins divided into two groups namely acidic and basic which are arranged in pairs. Keratins and certain keratin associated proteins are useful as markers of differentiation because their expression is both region and differentiation specific.
Historically, important discoveries in regard to keratin were made in the 1970’s. One was the finding of the spontaneous self assembly and polymerization of keratin filaments from denatured, soluble keratin proteins by dialysis in vitro.6 Different types of keratin were subsequently discovered using various methods. The advances in laboratory diagnostics have also aided in easier identification and characterization of keratin. Currently, the different types of keratin and their associated proteins serve as important markers of differentiation thus aiding in diagnosis of various pathological conditions. The keratin proteins have a uniform mode of distribution among the various layers of epithelium which gives an indication of the disease process. Also, various disorders are associated with defects in the keratin and their associated proteins which may manifest in skin or oral cavity or both.
Keratin
Keratins are defined as intermediate filament forming proteins with specific physicochemical properties produced in any vertebrate epithelia.4 They are multigene family of proteins constituting 85% of the total cellular protein in the cornified cells of the epidermis1 and encoded by a family of approximately 30 proteins.7
Structure of Keratin
Each keratin is characterized by a chain of amino acids as the primary structure, which varies in the number and sequence of amino acid as well as in polarity, charge and size. The amino acid sequence of a keratin influences the properties and function of the keratin filament.8 Furthermore, the position of a particular amino acid within the chain of amino acids can influence the entire architecture of the keratin molecule.9 The primary amino acid sequence is slightly longer than that of the mature keratin which indicates a post-translational modification of the keratin prior to the formation of keratin filaments.10 Post translational modifications such as the formation of disulphide bonds, phosphorylation, glycosylation, deamination or inter- and intra-chain peptide bonds can influence the conformation of the molecule and formation of keratin filaments.4
Keratin filaments have a tripartite secondary structure consisting of an N-terminal head domain, a central α-helical rod domain and C-terminal tail domain and all the proteins are able to self assemble into filaments.11 Each of these domains is divided into sub-domains. The head domain consists of sub-domains V1 and H1. The central α-helical rod domain is composed of sub-domains 1A, 1B, 2A, and 2B connected by linkers L1, L12 and L2. The tail domain is made of sub-domains H2 and V2.12 Domains and sub-domains are determined by the amino acid sequence of the keratin and serve various functions in the assembly of keratin filaments and in the binding of keratins and keratin filaments to cell adhesion complexes or to signalling molecules.13 The tertiary structure of keratins is a heterodimer that is formed by the rod domains of one acidic and one basic keratin in parallel orientation.14 Keratins are characterized by the capacity to form filaments with a complex quarternary structure including the formation of a tetramer, an octamer, and unit length filaments.4
The α-helical rod domain is critical for the assembly of intermediate filament proteins into filaments and contains all the information necessary for assembly, while the variant end domains play accessory roles in filament assembly and interaction with other proteins and cell structures.1
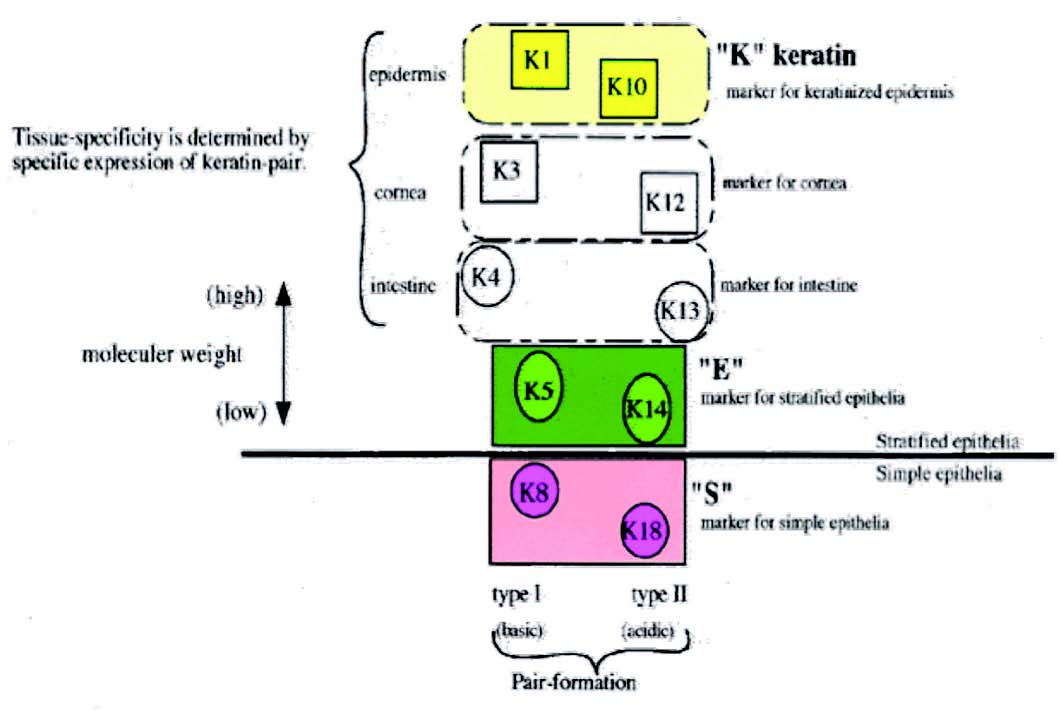
Figure 1: Distribution of keratin.
Different types of keratins are distinguished according to various characteristics such as physicochemical properties or according to cells and tissues that produce certain keratin. The keratins are broadly divided into4:
1. Primary keratins are those keratins which are always synthesized by the epithelial cells on a regular basis, e.g., K8/18 in simple epithelia, K5/14 in stratified epithelia.
2. Secondary keratins are those types of keratins which are produced by the epithelial cells in addition to or instead of primary keratins, e.g., K7/19 in simple epithelia, K15, and K6/16 in stratified epithelia.
During epidermal differentiation, low molecular weight keratins 5/14 of the basal layer are replaced by high molecular weight keratins 1/10,11 which can be used as a marker of keratinization.
Other types of keratin include:
1. Based on distribution
• Soft keratin: Found in the epidermis of skin in the form of flattened non-nucleated scales that slough continually. The disulfide links are fewer in number which allows some stretching but returns to normal upon relaxation of tension.
• Hard keratin: These are mainly found in nail, hair cortex, hair cuticle; the keratin type seen at these sites have very little flexibility owing to the presence of many cysteine disulfide crosslinks. They differ from the epithelial keratins by their considerably higher sulfur content in their non-helical head and tail domains, which is mainly responsible forn the high degree of filamentous cross-linking by keratin associated proteins.5
2. Based on X-ray diffraction pattern
• Alpha: The X-ray diffraction pattern of this type resembles that of α-helix with a 5.1 Å spacing. The α-helix is right handed and has 3.6 residues per turn. The hydrogen bonding occurs within one polypeptide chain.
• Beta: In the X-ray diffraction pattern of this type, periodic repeats were 3.5 and 7 angstroms. The helix is right-handed with an average of 6 residues. The hydrogen bonding occurs between neighboring polypeptide chains.
• Feather keratins
• Amorphous keratins
3. Based on amino acid sequence, keratins are classified into type I and type II
• Type I family includes keratins numbered 9- 20 which are composed of acidic proteins, with a molecular weight 40-56 kDa and pI- 4.9-5.4.
• Type II family includes keratins numbered 1- 8 which are composed of basic proteins, with a molecular weight of 52-67 kDa and pI- 6.5-8.5
4. Based on molecular weight2
• Low molecular weight keratins: Include keratins with a molecular weight of 40kDa. These keratins are mainly distributed in glandular and simple epithelia.
• Intermediate molecular weight keratins: Include keratins with a molecular weight intermediate between 40kDa and 57kDa and are found in stratified epithelia.
• High molecular weight keratins: Include keratins with a molecular weight of 57kDa and are seen in keratinized stratified epithelia
Functions of Keratins
• Keratins fundamentally influence the architecture and mitotic activity of the epithelial cells.15
• Keratins and associated filaments provide a scaffold for epithelial cells and tissues to sustain mechanical stress, maintain their structural integrity, ensure mechanical resilience, to protect against variations in hydrostatic pressure and establish cell polarity.16
• Keratins and its filaments are involved in cell signalling, cell transport, cell compartmentalization and cell differentiation.17
• Keratin filaments also influence cell metabolic processes by regulating protein synthesis and cell growth.18
• Keratins may also be involved in the transport of membrane bound vesicles in the cytoplasm of the epithelial cells.
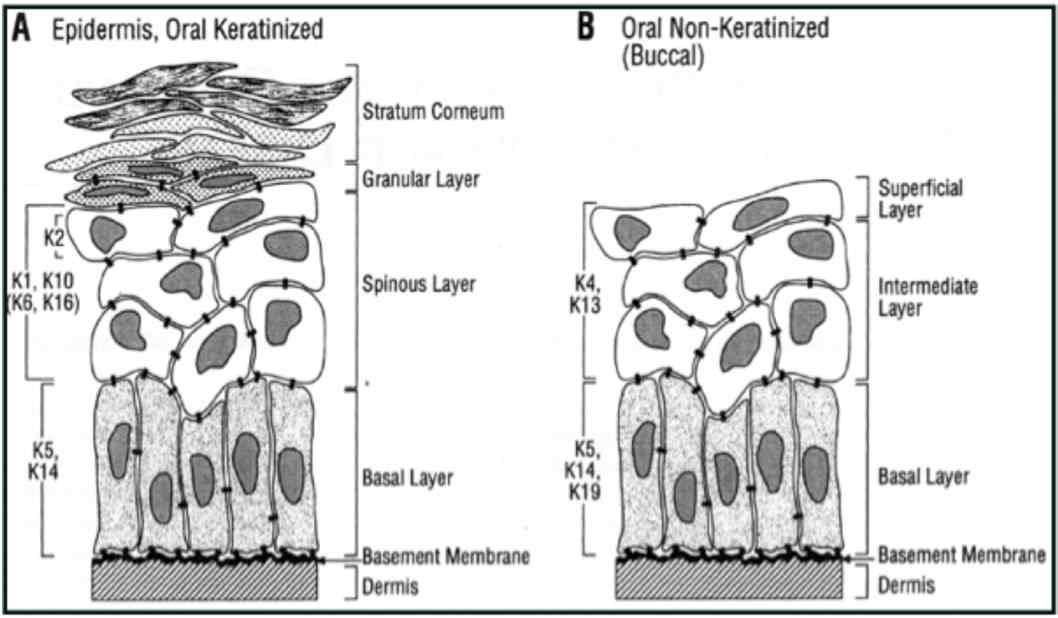
Figure 2: Distribution of keratin in oral epithelium (Reproduced from Presland and Dale 2000).1
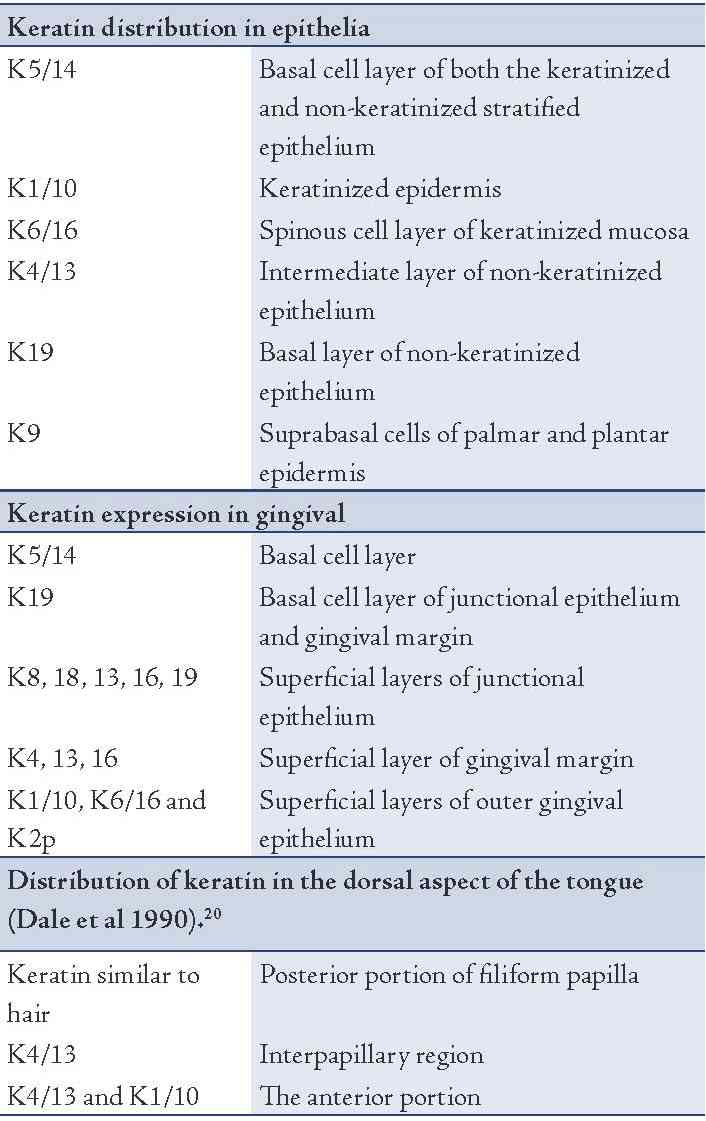
In general, low molecular weight keratins are expressed in glandular and simple epithelia, intermediate molecular weight keratins are expressed in stratified epithelia and high molecular weight keratins are expressed in keratinized stratified epithelia. K8/18 is the primary keratin and K7 is the secondary keratin of the simple epithelium.19 The expression of major proteins in the various layers of epithelia, gingiva and dorsal aspect of tongue is given in Table 1.
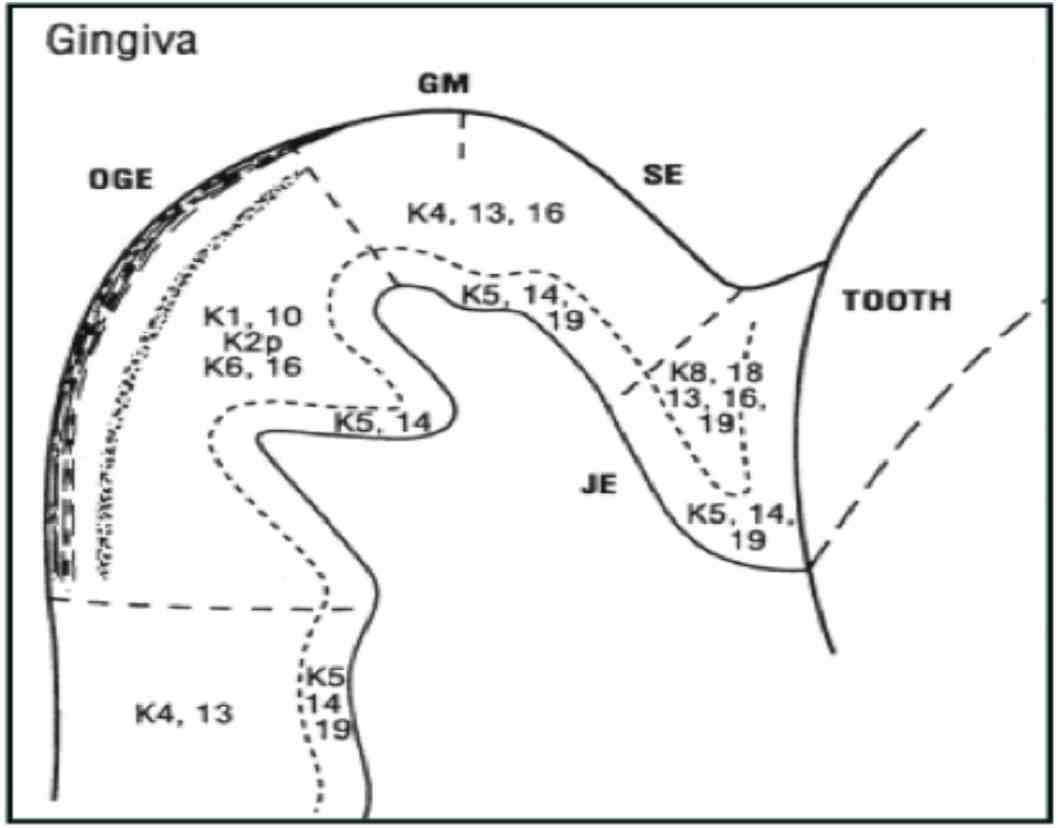
Figure 3: Distribution of keratin in gingival epithelium (Reproduced from Presland and Dale 2000).1
Table 1: Distribution of major keratins.
Certain basic keratins are minor constituents of the cytoskeleton that are expressed in the suprabasal cells of various stratified soft-keratinizing epithelia. K76 is produced in the upper layers of the stratified epithelium of the hard palate and gingiva.21 K78 is produced in the cells of the epithelial covering of the human tongue while K80 is produced in the suprabasal cells of the epithelium of the human tongue.22 Merkel cells which functions as touch receptors express K8/18, K19 and K20. K19 is the smallest keratin as it lacks the typical tail domain. K26 is exclusively specific to taste bud anlagen in developing oral mucosa.23 K24 is sometimes expressed in the epithelium of tongue. Myoepithelial cells were found to express K17.24
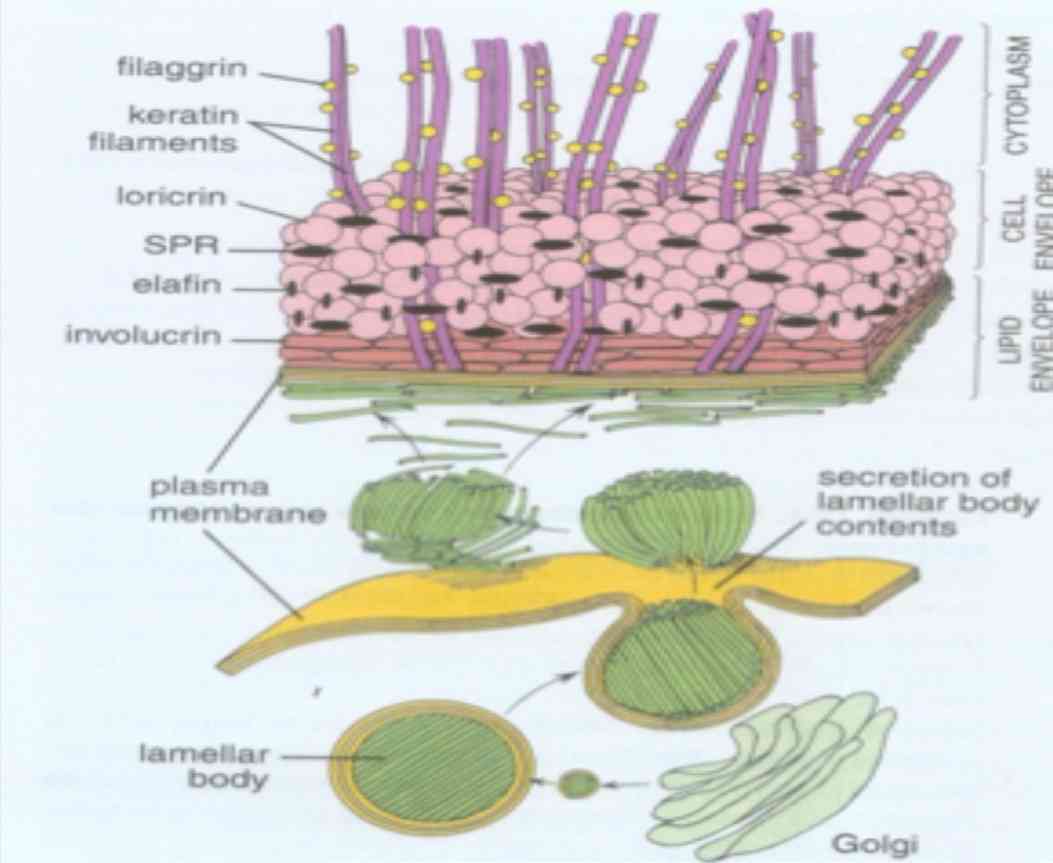
Figure 4: Schematic representation of various keratin associated proteins (Reproduced from Textbook of Histology, Ross and Paulina 5th edition).25
The keratin filament associated proteins (KFAP) are non-filamentous, structural proteins that are produced in the keratinocytes of the stratum granulosum of stratified keratinizing and cornifying epithelia and stored in keratohyalin granules.26 KFAP’s also known as intermediate filament associated proteins are composed of filaggrin, tricohyalin, desmosomal proteins and proteins of cornified cell envelope (loricrin, involucrin and small proline rich proteins).20 Two types of KFAP’s can be distinguished based on the structures to which they contribute; the KFAP’s such as loricrin and involucrin that are stored in a subset of keratohyaline granules form the subcytolemmal cornified cell envelope and the KFAP’s that are stored in another subset of keratohyaline granules that combine with keratin filaments to form the filament matrix complex stabilizing the cytoskeleton of cornifying keratinocytes.27
Filaggrin is a cationic protein which aids in dense packing of keratin within a cornified cell layer. They are synthesized in the granular cell layer as a large precursor molecule (profilaggrin), stored in keratohyaline granules and converted to filaggrin upon transition of granular cells to fully differentiated cornified cells.20 They facilitate disulfide bond formation between keratin polypeptide chains. They may also play a role in maintaining the osmolarity and flexibility of epidermis.28 Expression of filaggrin is enhanced in conditions of hyperkeratinization and thus serve as useful markers to distinguish non-keratinized from keratinized epithelia.
Tricohyalin is expressed in keratinizing filiform papilla of tongue, nail matrix, new born foreskin epidermis and in isolated cells of normal adult epidermis.29 These are single stranded α-helical rod that binds keratin intermediate filaments by ionic interactions. They function as intercellular cement as well as cross bridging proteins. It may play a role in sequestering calcium and thus regulate the activity of calcium dependent enzymes.1 Desmosomal proteins link epithelial cells to each other. They help in attachment of keratin intermediate filament cytoskeleton to cell surface. They are inclusive of integral proteins (desmoglein and desmocollin), cytoplasmic adapter proteins (desmoplakin and plakoglobin) and plaque associated proteins (plakophilin, envoplakin, and periplakin).
Proteins of cornified cell envelope are 15nm thick cross linked sheath of protein deposited on the inner face of plasma membrane of keratinocytes. They are expressed in the suprabasal layer and act as a barrier function of stratified keratinized epithelium. The most abundant cell envelope proteins are loricrin, involucrin and small proline rich proteins. Loricrin is expressed in granular cells of epithelium and other keratinized epithelia such as gingiva and hard palate. It is mainly composed of glycine, serine, and cysteine. Involucrin is expressed in differentiating keratinocytes and consists of short polypeptide repeats rich in glycine, lysine and glutamine. Small proline rich proteins play a crucial role in epithelia that must be flexible.1
Molecular Understanding of KeratinIintermediate Filaments
Keratins are encoded by genes that possess a similar nucleotide sequence. A gene encoding a specific keratin is labeled KRT followed by the number designated to the specific keratin.30 In humans, keratins are encoded in 54 genes and these genes are clustered on 2 chromosomes. The acidic type I keratin genes are clustered on chromosome 17 and the basic type II genes are clustered on chromosome 12. All sequences contain TATA box (binding site of RNA polymerase II) about 30bp upstream from ATG translator initiator codon. A consensus sequence of AA(Pu)CCAAA sequence is found in almost all keratins and this sequence is presumably important for tissue specific expression by serving as a binding site for a specific factor in stratified epithelia.1
Factors Regulating Differentiation
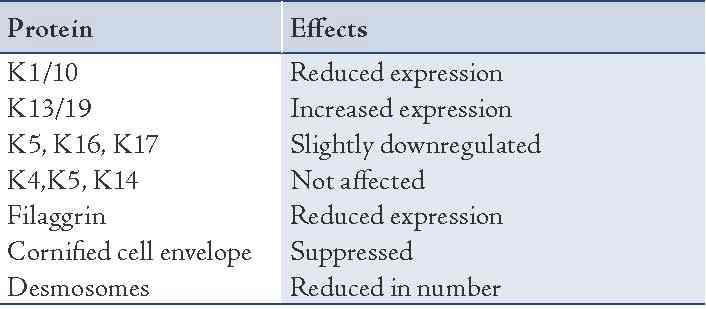
Various factors that regulate epithelial differentiation include the role of adjacent mesenchymal tissue, components of extracellular matrix, growth factors like epidermal growth factor, transforming growth factor alpha and beta, retinoids and calcium. The role of retinoids in epithelial differentiation is depicted in Table 2.
Table 2: Effects of retinoids on various keratin proteins.
Keratinization Disorders
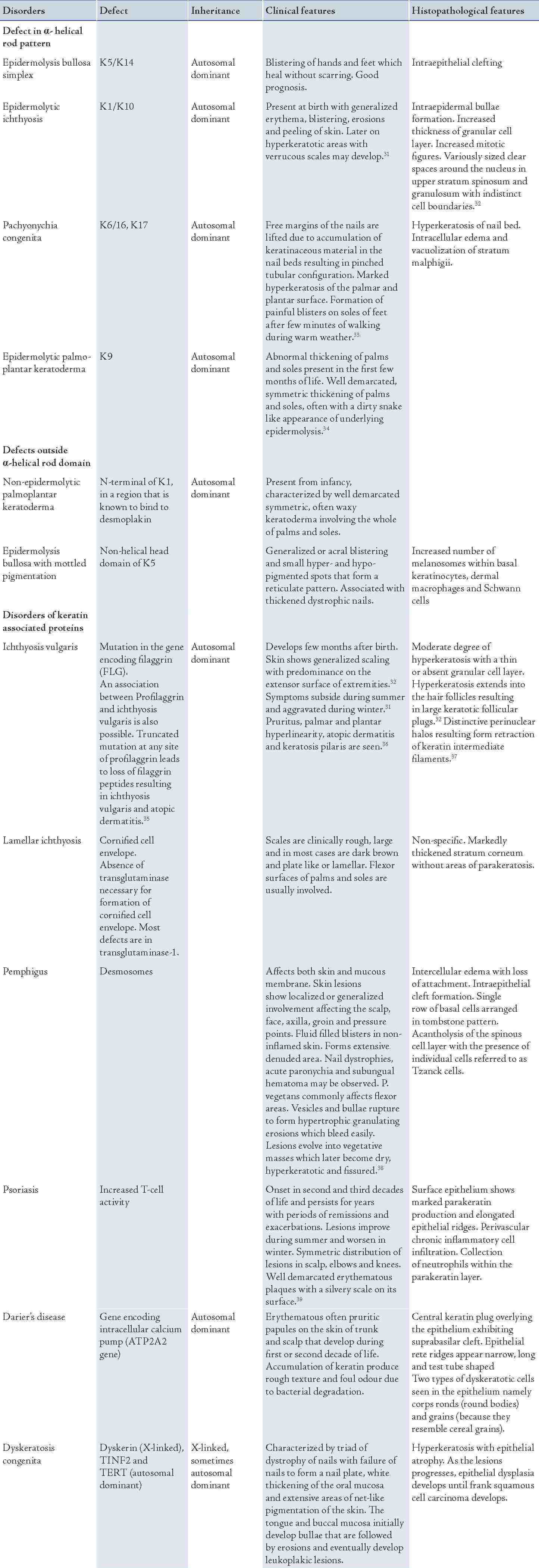
A wide range of disorders occur as a result of mutation in the gene encoding for the various keratin proteins. These disorders comprise of the lesions affecting the skin and mucous membrane depending on the distribution of keratin and certain disorders may present with both skin and oral manifestations. The disorders affecting the skin are presented in Table 3.
Table 3: Keratinization disorders of the skin.
Keratinization Disorders with Predominant/Associated Oral Lesions
WhiteSponge Nevus
White sponge nevus, also referred to as Cannon’s disease or familial white folded dysplasia, was first described by Cannon in 1935. The disease is inherited as an autosomal dominant trait and is characterized by benign leukokeratotic lesions of early onset with periods of remissions and exacerbations.40 It occurs due to a mutation in the gene encoding for K4/13 that is expressed in the spinous cell layer of non-keratinized mucosa of the oral cavity. The lesions appear in early childhood as symmetrical, thickened, white corrugated or velvety diffuse plaques affecting the buccal mucosa bilaterally. Other sites include the ventral aspect of tongue, labial mucosa, soft palate and floor of the mouth. Histopathology demonstrates prominent hyperkeratosis with marked acanthosis and clearing of cytoplasm in the spinous cell layer. An eosinophilic condensation is sometimes noted in the perinuclear region of cells in the superficial layers of the epithelium. The lesion follows an indolent course and has a good prognosis.39
Pachyonychia Congenita (PC) (Greek- thick nails from birth)
This is a rare autosomal dominant disorder caused by mutation in the gene encoding for K6/16 and K17 that typically affects the nails and palmo-plantar skin and often the oral mucosa, tongue, larynx, teeth and hair. The term was first described by Jadassohn and Lewandowski in 1906.41 There are two major subtypes termed as PC-1 (Jadassohn-Lewandowski type) and PC-2 (Jackson-Lawler type). Currently a new genotypic classification categorizes pachyonychia congenita as PC-K6a, PC-K6b, PC-K16 and PC-K17 corresponding to the mutation in the respective gene.42 The oral manifestations are more common in PC-1 type and manifest as thickened white plaques that involve the lateral margins and dorsal surface of the tongue. The tongue develops a white to yellow thickening that can mimic oral candidiasis, white sponge nevus or hairy tongue.41 Neonatal teeth have been reported in PC-2 although these individuals do not have oral white lesions. Hoarseness of voice and dyspnea may occur due to laryngeal mucosal involvement. Histopathology shows marked hyperkeratosis and acanthosis with perinuclear clearing of the epithelial cells.39
Dyskeratosis congenita (DC)
DC is a rare X-linked disorder first described by Zinsser in 1906 and later reported in detail by Engmen and Cole thus being referred to as Cole- Engmen syndrome or Zinsser-Cole-Engmen syndrome.43 The disease is sometimes inherited as an autosomal dominant disorder. The genes that undergo mutation include dyskerin (X-linked), TINF2, and TERT (autosomal dominant).44 DC occurs mostly in males between the age group of 5-12 years and is caused by mutation in DKC1 gene thus disrupting the normal maintainence of telomerase.39 This could be a fatal condition in which majority of the patients develop aplastic anemia and sometimes malignant transformation of the keratotic white patch may occur.43 The lesion is characterized by triad of dystrophy of nails with failure of nails to form a nail plate, white thickening of the oral mucosa, and extensive areas of net-like pigmentation of the skin.32 Both the hard and soft tissues of oral cavity are affected by DC. The tongue and buccal mucosa initially develop bullae that are followed by erosions and eventually develop leukoplakic lesions. Severe periodontal destruction may occur due to anomalies in ectodermally derived structures and diminished host response caused by neutropenia.45 Biopsy of the oral lesions shows hyperkeratosis with epithelial atrophy. As the lesions progresses, epithelial dysplasia develops until frank squamous cell carcinoma develops. Prognosis is unpredictable and sufficient follow-up is required.
Hereditary Benign Intraepithelial Dyskeratosis
Also known as Witkop-Von Sallman syndrome; this condition is a rare autosomal dominant disorder which frequently affects the oral and conjunctival mucosa. The lesion was first reported in 1959 by Von Sallmann and Paton. The condition predominantly develops in early childhood and manifests as thick white corrugated plaques involving the buccal and labial mucosa. Superimposition of candidal infection may occasionally occur. Histopathological features include prominent parakeratin production in addition to marked acanthosis. A peculiar dyskeratotic process is scattered throughout the upper spinous cell layer of oral epithelium giving an appearance of cell within a cell phenomenon.46
Darier’s Disease (Keratosis Follicularis)
This condition is predominantly a skin lesion with subtle oral lesions. Skin lesions may first appear as yellow-brown papules commonly in forehead, scalp, back and chest. Involvement of the hand is very common and the lesions include punctate keratosis, palmar pits and hemmorhagic macules. The oral lesions are asymptomatic and consist of multiple normal colored or white flat topped papules which if numerous gives rise to cobble-stone appearance primarily affecting the hard palate and alveolar mucosa. Warty dyskeratoderma is an uncommon solitary lesion occurring in skin or oral mucosa which has histopathological similarity to Darier’s disease and hence is referred to as isolated Darier’s disease. They typically present as a solitary, asymptomatic, pink or white umblicated papule on keratinized mucosa especially the hard palate and alveolar ridges. A warty or rough surface is noted in some lesions.
Pemphigus
This is a group of life threatening autoimmune mucocutaneous diseases and is mentioned among the group of keratinization disorders primarily because of the defects in keratin associated protein, desmosomes. The proteins associated with desmosomes namely desmoglein 1 and 3 are affected in this condition wherein auto-antibodies are directed against these proteins. Of the various types, pemphigus vulgaris and vegetans are commonly noticed in the oral cavity. P. vulgaris appears as painful ulcers on buccal mucosa, palate, gingival, or dorsum of tongue which eventually spread. The lesions are covered by slough and have ragged edges. P. vegetans appear as hypertrophic granulating erosions which evolve into vegetative masses that later become dry, hyperkeratotic, and fissured. Treatment mainly involves corticosteroid administration (both local and systemic) along with other immunosuppressive agents and supportive therapy.47-49
Keratinizing Lesions of Oral Cavity
In addition to the lesions occurring primarily due to defect in keratinization, certain lesions exist which histopathologically demonstrate hyperkeratosis irrespective of the etiological factor. Such lesions are collectively referred to as keratinizing lesions of the oral cavity and are inclusive of reactive lesions (frictional keratosis, smokeless tobacco induced keratosis, nicotine stomatitis, hairy tongue, hairy leukoplakia), immune mediated lesions (lichen planus, discoid lupus erythematosus, graft versus host disease), pre-neoplastic and neoplastic diseases (actinic cheilosis, leukoplakia, proliferative verrucous leukoplakia, verrucous carcinoma, squamous cell carcinoma), and infections (squamous cell papilloma, verruca vulgaris, condyloma accuminatum, molluscum contagiosum and verruciform xanthoma).
Clinical Significance
Keratin expression patterns are characteristic for distinct stages during cellular epithelial differentiation from embryonal to adult and of the internal maturation program during development. Epithelial tumors including metastasis most widely retain their keratin patterns of their normal origin; thus the determination of the keratin patterns of tumors is widely exploited for cell and tumor typing. Therefore keratins have evolved to be one of the most potent epithelial differentiation and tumor markers in cell biology, embryology, and surgical pathology. Specific antibodies against several keratins are routinely used for immunohistochemical typing of carcinoma in tumor diagnostics.
Keratins can be used as differentiation markers in normal oral epithelia. K8/18 serve as markers for simple epithelial differentiation, K1/10 are markers for keratinized epithelium, K4/13 can be used as markers for non-keratinized epithelium. K6/16 are considered as hyperproliferative markers which are expressed in sites of high epidermal keratinocyte turnover and in pathological hyperproliferative conditions affecting the skin.50
In general, K8/18 is found to strongly stain in most adenocarcinoma, hepatocellular carcinoma, renal cell carcinoma, and neuroendocrine carcinoma. Another clinical application is the monitoring of fragments of these keratins in the serum as serological tumor markers to monitor cancer load, cancer progression, and response to therapy.5 The detection of soluble K19 fragments in the serum released by carcinoma cells by the CYFRA 21-1 has found broad clinical application as a marker to monitor treatment and evaluate response to therapy.51 K20 is a potent immunohistochemical marker in tumor pathology since its peculiar expression spectrum is essentially maintained in the corresponding primary and metastatic carcinoma.52 K20 positivity is seen in majority of gastric adenocarcinoma, transitional cell carcinoma, and Merkel cell carcinoma.53 K20 is considered to be a consistent marker for Merkel cell carcinoma.
K6 and 16 are typically and strongly expressed in squamous cell carcinoma of different sites. K6, 16 and 17 are inducible keratin upon stress, injury and inflammation and therefore increased expression is seen in squamous cell carcinoma. The increased expression of K17 which is usually absent in normal epithelia could be attributed to neo-expression during tumorigenesis.54 A unique feature of K17 is its inducibility after skin injury. After K6/16, K17 is switched on in regenerating and migrating epidermal keratinocytes upon wound healing.55
In the oral cavity, there is increased expression of K1/10, K4/13 in well-differentiated oral squamous cell carcinomas. Less well-differentiated oral squamous cell carcinoma express neither of these keratin pairs to any significant extent but do express K19. Moderated and poor oral squamous cell carcinoma may express K8 and sometimes K7 and 18. The expression of K8 and K18 in oral squamous cell carcinoma is considered to be an independent prognostic marker and indicates a decreased overall and progression free survival.56 Also K5 and K6 are considered as useful markers for squamous cell carcinomas in histologically uncertain, poorly differentiated or metastatic tumor cases.54 Squamous cell carcinomas are strikingly positive for CK14 which is a distinctive feature to differentiate from high grade mucoepidermoid carcinoma where CK 14 is only focally positive. Mucoepidermoid carcinoma also expresses CK7, 8, and 19 and negative for CK10. In areas of squamous metaplasia seen in both squamous cell carcinoma and mucoepidermoid carcinoma, SCC is positive for CK10 and MEC for CK13.57 CK10 is usually absent in these tumors, reflecting that true keratinization is an uncommon finding in mucoepidermoid carcinoma. CK13 is also found to be useful in differentiating mucoepidermoid carcinoma from other salivary gland tumors.58 Consistent expressions of K13 and K19 may provide a useful marker of odontogenic epithelium in general.59 K19 scores are found to reflect histological differentiation as well as predicting the clinical outcome. Combining K19 immunostaining with regular H & E stain may be helpful to facilitate and assure assigning a more accurate grade for oral epithelial dysplasia.60
Conclusion
Keratins and keratinization has been the subject of research for a long time owing to the various disorders of keratinization affecting the skin and oral cavity and also the role of keratins in tumor diagnosis. Considerable progress in this field has been achieved in the recent past courtesy to the advancement in technologies helpful in determining the keratin pattern and expression. Knowledge of the expression patterns of various keratin markers; their distribution and functions plays a vital role in understanding of the tumor pathology. Also the identification of keratin antibodies as markers of diagnostic pathology has received tremendous boost and is a specialized area of interest currently. Thus a thorough knowledge regarding the various aspects of keratin will be helpful in diagnosis and treatment of various keratinization disorders and also contribute to diagnostic and prognostic significance in tumor pathology.
Acknowledgements
The authors reported no conflict of interest and no funding was received in this work.
References
1. Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med 2000;11(4):383-408.
2. Nanci A. Tencate’s Oral Histology 2003; 6th edtn, Elsevier: 336-48.
3. Shroeder HE. Differentiation of human oral epithelia 1981. Krager, Basel.
4. Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 2009 Apr;214(4):516-559.
5. Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol 2008 Jun;129(6):705-733.
6. Steinert PM, Idler WW, Zimmerman SB. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol 1976 Dec;108(3):547-567.
7. Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, et al. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol 1981 Dec;153(4):933-959.
8. Roop DR, Chang CK, Titterington L, Meyers CA, Stanley JR, Steinhart PM, et al. Syntehtic peptides corresponding to keratin subunits elicit highly specific antibodies. J Biochem 1984;259:8037-8040.
9. Wu KC, Bryan JT, Morasso MI, Jang SI, Lee JH, Yang JM, et al. Coiled-coil trigger motifs in the 1B and 2B rod domain segments are required for the stability of keratin intermediate filaments. Mol Biol Cell 2000 Oct;11(10):3539-3558.
10. Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci 2001 Jul;114(Pt 14):2569-2575.
11. Coulombe PA, Tong X, Mazzalupo S, Wang Z, Wong P. Great promises yet to be fulfilled: defining keratin intermediate filament function in vivo. Eur J Cell Biol 2004 Dec;83(11-12):735-746.
12. Lane EB, McLean WH. Keratins and skin disorders. J Pathol 2004 Nov;204(4):355-366.
13. Hatzfeld M, Burba M. Function of type I and type II keratin head domains: their role in dimer, tetramer and filament formation. J Cell Sci 1994 Jul;107(Pt 7):1959-1972.
14. Er Rafik M, Doucet J, Briki F. The intermediate filament architecture as determined by X-ray diffraction modeling of hard alpha-keratin. Biophys J 2004 Jun;86(6):3893-3904.
15. Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res 2007 Jun;313(10):2021-2032.
16. Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 2002 Feb;14(1):110-122.
17. Vaidya MM, Kanojia D. Keratins: markers of cell differentiation or regulators of cell differentiation? J Biosci 2007 Jun;32(4):629-634.
18. Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol 2007 Feb;19(1):13-23.
19. Pekny M, Lane EB. Intermediate filaments and stress. Exp Cell Res 2007 Jun;313(10):2244-2254.
20. Dale BA, Salonen J, Jones AH. New approaches and concepts in the study of differentiation of oral epithelia. Crit Rev Oral Biol Med 1990;1(3):167-190.
21. Rogers MA, Winter H, Langbein L, Bleiler R, Schweizer J. The human type I keratin gene family: characterization of new hair follicle specific members and evaluation of the chromosome 17q21.2 gene domain. Differentiation 2004 Dec;72(9-10):527-540.
22. Rogers MA, Edler L, Winter H, Langbein L, Beckmann I, Schweizer J. Characterization of new members of the human type II keratin gene family and a general evaluation of the keratin gene domain on chromosome 12q13.13. J Invest Dermatol 2005 Mar;124(3):536-544.
23. Witt M, Kasper M. Distribution of cytokeratin filaments and vimentin in developing human taste buds. Anat Embryol (Berl) 1999 Apr;199(4):291-299.
24. Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol 2001 May;116(5):633-640.
25. Ross MH, Pawlina W. Histology: A text and atlas. With correlated cell and molecular biology. 5th ed 2006. Lippincott Williams and Wilkins.
26. Jessen H. Two types of keratohyalin granules. J Ultrastruct Res 1970 Oct;33(1):95-115.
27. Eckert RL, Sturniolo MT, Broome AM, Ruse M, Rorke EA. Transglutaminase function in epidermis. J Invest Dermatol 2005 Mar;124(3):481-492.
28. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem 1995 Jul;270(30):17702-17711.
29. Hamilton EH, Payne RE Jr, O’Keefe EJ. Trichohyalin: presence in the granular layer and stratum corneum of normal human epidermis. J Invest Dermatol 1991 May;96(5):666-672.
30. Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, et al. New consensus nomenclature for mammalian keratins. J Cell Biol 2006 Jul;174(2):169-174.
31. Uitto J, Richard G, McGrath JA. Diseases of epidermal keratins and their linker proteins. Exp Cell Res 2007 Jun;313(10):1995-2009.
32. Elder DE, Elenitsas R, Johnson BL, Murphy GF. Lever’s histopathology of skin. 9th edtn. Chapter 6- Congenital diseases (Genodermatoses). Edited by Johnson JR, Honig P. Lippincott Williams & Wilkins 2005:139-77.
33. Shimizu H. Shimizu’s text book of dermatology. Chapter 5. Disorders of abnormal keratinization. Hokkaido University Press 2005:229-37.
34. Freedberg MI, Eisen A, Wolff K, Austen FK, Goldsmith LA, Katz S. Fitzpatrick’s Dermatology in general medicine. 6th edtn; 2003. McGraw Hill.
35. Osawa R, Akiyama M, Shimizu H. Filaggrin gene defects and the risk of developing allergic disorders. Allergol Int 2011 Mar;60(1):1-9.
36. Akdeniz N, Karadag AS, Calka O, Ediz L, Karadag R, Celen I. A case report of icthyosis vulgaris with arthropathy and ophthalmic findings. J Turk Acad Dermatol 2011;5(3):1153-1156.
37. Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol 2011 May;178(5):2252-2263.
38. Burns DA, Breathnach SM, Cox N & Griffiths CE. Rook’s Text book of dermatology. 7th edtn. Wiley Blackwell 2004; 3:41.1-41.24.
39. Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 3rd edtn. Elsevier Saunders 2009:741-815.
40. Marcushamer M, King DL, McGuff S. White sponge nevus: case report. Pediatr Dent 1995 Nov-Dec;17(7):458-459.
41. Leachman SA, Kaspar RL, Fleckman P, Florell SR, Smith FJ, McLean WH, et al. Clinical and pathological features of pachyonychia congenita. J Investig Dermatol Symp Proc 2005 Oct;10(1):3-17.
42. McLean WH, Hansen CD, Eliason MJ, Smith FJ. The phenotypic and molecular genetic features of pachyonychia congenita. J Invest Dermatol 2011 May;131(5):1015-1017.
43. Auluck A. Dyskeratosis congenita. Report of a case with literature review. Med Oral Patol Oral Cir Bucal 2007 Sep;12(5):E369-E373.
44. Sasa GS, Ribes-Zamora A, Nelson ND, Bertuch AA. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin Genet 2012 May;81(5):470-478.
45. Yavuzyilmaz E, Yamalik N, Yetgin S, Kansu O. Oral-dental findings in dyskeratosis congenita. J Oral Pathol Med 1992 Jul;21(6):280-284.
46. Allingham RR, Seo B, Rampersaud E, Bembe M, Challa P, Liu N, et al. A duplication in chromosome 4q35 is associated with hereditary benign intraepithelial dyskeratosis. Am J Hum Genet 2001 Feb;68(2):491-494.
47. Scully C, Challacombe SJ. Pemphigus vulgaris: update on etiopathogenesis, oral manifestations, and management. Crit Rev Oral Biol Med 2002;13(5):397-408.
48. Black M, Mignogna MD, Scully C, Number II. Number II. Pemphigus vulgaris. Oral Dis 2005 May;11(3):119-130.
49. Robinson JC, Lozada-Nur F, Frieden I. Oral pemphigus vulgaris: a review of the literature and a report on the management of 12 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997 Oct;84(4):349-355.
50. Morgan PR, Shirlaw PJ, Johnson NW, Leigh IM, Lane EB. Potential applications of anti-keratin antibodies in oral diagnosis. J Oral Pathol 1987 Apr;16(4):212-222.
51. Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin Biochem 2004 Jul;37(7):529-540.
52. Moll R, Lowe A, Laufer J, Franke WW. CK 20 in human carcinoma. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 1992;14(2):427-447.
53. Goldstein NS, Bosler DS. Immunohistochemistry of the gastrointestinal tract, pancreas, bile ducts, gallbladder and liver. In: Dabbs DJ; Diagnostic immunohistochemistry 2006. Churchill Livingstone, Elsevier:442–508
54. Moll R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem 1998;31:205-262.
55. Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol 1996 Feb;132(3):381-397.
56. Fillies T, Werkmeister R, Packeisen J, Brandt B, Morin P, Weingart D, et al. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer 2006;6:10.
57. Sobral AP, Loducca SV, Kowalski LP, Santos IR, Almeida OP, Araújo NS, et al. Immunohistochemical distinction of high-grade mucoepidermoid carcinoma and epidermoid carcinoma of the parotid region. Oral Oncol 2002 Jul;38(5):437-440.
58. Pires FR, Chen SY, da Cruz Perez DE, de Almeida OP, Kowalski LP. Cytokeratin expression in central mucoepidermoid carcinoma and glandular odontogenic cyst. Oral Oncol 2004 May;40(5):545-551.
59. Matthews JB, Mason GI, Browne RM. Epithelial cell markers and proliferating cells in odontogenic jaw cysts. J Pathol 1988 Dec;156(4):283-290.
60. Safadi RA, Musleh AS, Al-Khateeb TH, Hamasha AA. Analysis of immunohistochemical expression of k19 in oral epithelial dysplasia and oral squamous cell carcinoma using color deconvolution-image analysis method. Head Neck Pathol 2010 Dec;4(4):282-289.
|