Perivascular epithelioid cell tumors (PEComas) are a family of rare mesenchymal tumors closely related to angiomyolipomas, lymphangiomyomatosis, and clear cell sugar tumors of the lung. They have no known benign cell counterpart but are made up of perivascular epithelioid cells displaying both melanocytic and smooth muscle differentiation with specific histological and immunological features.1 PEComas have been described in the literature originating from various sites such as the pelvis, genital tract, lung, and intra-abdominal organs with cutaneous or subcutaneous PEComas exceptionally rare among these tumors.2 There is a paucity of literature describing PEComas over the abdominal wall. Here we present a rare case of malignant subcutaneous PEComas over the abdomen, the first described in Southeast Asia.
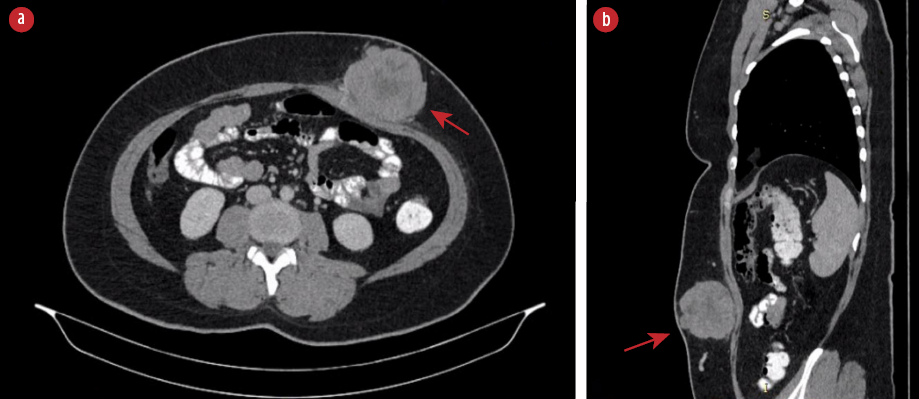
Figure 1: (a) CT scan of the abdomen showed a heterogeneous lobulated subcutaneous lesion (red arrow) at the left anterior abdominal wall in axial view. (b) The lesion (red arrow) at another angle in sagittal view.

Figure 2: (a) Preoperative marking depicting the mass and incision. (b) Mesh repair of the rectus sheath defect post excision of the tumor. (c) Posterior surface of the resected specimen, showing the rectus sheath resected along with the tumor. (d) Side view of the resected specimen.
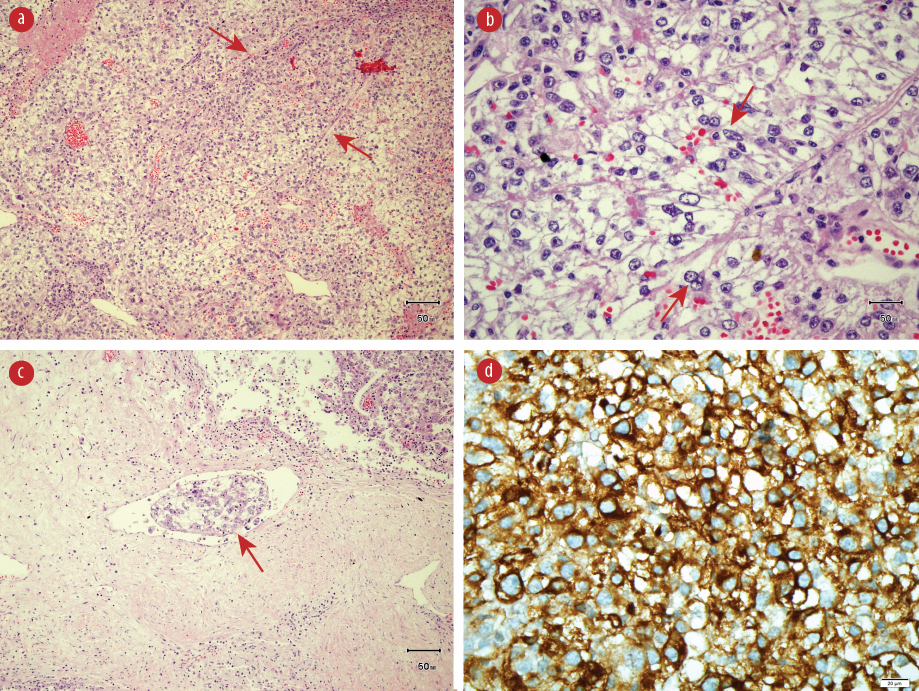
Figure 3: (a) Hematoxylin and eosin (H&E) staining showing epithelioid cells in nests with thin fibrovascular septae (red arrows), magnification = 20 ×. (b) H&E staining showing epithelioid cells (red arrows), magnification = 40 ×. (c) H&E staining showing lymphovascular invasion (red arrow), magnification = 20 ×. (d) Epithelioid cells with positive staining with human melanoma black-45, magnification = 40 ×.

Figure 4: Immunohistochemistry revealed negative staining for (a) desmin, (b) Melan-A, (c) PanCKAE1/AE3, and (d) U79 RCC marker. Magnification = 40 ×.
Case report
A 34-year-old healthy woman presented with a rapidly enlarging anterior abdominal wall mass noticed over three months. She was otherwise well apart from occasional pain over the lesion. There was no family history of similar conditions. Physical examination revealed a large subcutaneous mass that was firm and mobile with increased prominence upon muscle contraction. The overlying skin was normal. An ultrasound showed a solid lobulated mass with vascularity indenting the abdominal wall. A tru-cut biopsy revealed inflamed fibrocollagenous tissue infiltrated by epithelioid tumor cells. A computed tomography (CT) scan of the abdomen showed a heterogeneous lobulated subcutaneous lesion at the left anterior abdominal wall measuring 54 × 73 × 68 mm with central necrosis [Figure 1]. There was no calcification. There was a poor plane of demarcation with the abdominal muscles but no evidence of distant metastasis.
She was elected for a wide local excision, which was performed under general anesthesia as she has an acceptable anesthetic risk. An elliptical incision was made on a previously made skin marking [Figure 2a]. Intraoperatively, there was a mobile tumor measuring 8 × 6 cm with the posterior part attaching to the anterior rectus sheath. A wide local excision with 1 cm margin was performed, resecting along the involved anterior rectus sheath. The feeding vessel is from the superior epigastric artery, and it was carefully identified, doubly ligated, and divided. A non-degradable polypropylene mesh was incorporated on the breached segment to avoid future herniation [Figure 2b]. A vacuum drain was inserted to avoid the risk of deep surgical site infection from hematoma collection.
The resected specimen revealed a solitary tumor measuring 75 × 55 × 90 mm [Figure 2c and 2d]. The cut section of the tumor showed a friable lesion with golden yellow tan orange cut surface and hemorrhage area. All resection margins were tumor-free, with the closest margin of 5 mm at the deep margin. Histology demonstrated epithelioid cells in nests with rounded nuclei, irregular nuclear membrane, and abundant eosinophilic to clear cytoplasm with thin fibrovascular septa and some spindle cells with evidence of lymphovascular invasion [Figure 3a-3c]. It showed moderate to marked nuclear atypia and nuclear pleomorphism with many large and bizarre nuclei. Less than 50% of coagulative necrosis was observed with mitotic figures (3 mitoses/10 high power fields (HPFs)). Immunohistochemistry (IHC) revealed that the tumor cells were positive for human melanoma black (HMB)-45 [Figure 3d], but negative for desmin [Figure 4a], myogenin, Melan-A [Figure 4b], PanCKAE1/AE3 [Figure 4c], U79 RCC marker [Figure 4d], S-100, vimentin, smooth muscle actin, and CD10. This tumor was graded as the French Federation of Cancer Centers Sarcoma Group grade 2. She was discharged on day three post-operatively and completed 50 Gy/25 fractions (2 Gy per fraction) of adjuvant radiotherapy. She is currently well with no signs of recurrence.
Discussion
Most cases of PEComas have been reported to occur in females with a median age of 43 years old which shows a strong female predominance despite excluding those of gonadal origin. As mentioned above, cutaneous or subcutaneous PEComas are rare, with only 48 cases reported to date. Of these, only three cases, including ours, are completely subcutaneous with no dermal involvement.1,3–5 Most of these tumors measure around 10–20 mm, making ours by far the largest to date.
Cutaneous PEComas characteristically present in a well-demarcated dermal lesion composed of epithelioid cells with a large, clear or slightly granular cytoplasm, and nuclei arranged in a nested or trabecular pattern intermingled with spindle cells. These cells are arranged within fine vasculature ranging from capillaries to thicker arterioles and arteries.6 Various cellularities are described with low to moderate cellularity, the commonest of all. Several cases have also described multinucleated giant cells with a central zone of eosinophilia which is not found in our case. An important histological finding to note is the mitotic activity which can range from 0 to 50 mitoses/50 HPF as atypical mitotic figures are only present in a minority of cases. Some tumors have coagulative cell necrosis, which may be representative of the larger-sized tumors.7
The characteristic IHC of PEComas has contributed to diagnosing soft tissue tumors, which expresses at least one melanocytic marker. They most commonly are positive for HMB-45 as in our case and sometimes are positive for Melan-A, tyrosinase, and microphthalmia transcription factor.6 Approximately half display positivity for muscle markers such as actin, desmin, vimentin, myoglobin, and myosin. Calder et al,8 described the negativity of S-100 in PEComas to differentiate between melanoma; however, 33% of cases were said to be positive for S-100 in a case series reported by Folpe et al.7 To date, there have only been three cases describing a negative expression of PEComas to HMB-45.9
The majority of cutaneous PEComas are located at the extremities and display benign features. However, it appears that those on the scalp, face, and trunk (as with ours) are more likely to display malignant features.3,4,8,10 Folpe et al,7 proposed a classification of PEComas to define the criteria of malignancy in 2005 and classified PEComas into either ‘benign’, ‘uncertain malignant potential’, or ‘malignant’. This was derived from the strong association of several features to recurrences and metastasis which includes tumor size of > 5 cm, infiltrative growth pattern, high nuclear grade, necrosis and mitotic activity of > 1 mitoses/50 HPF. It was then built on by Bleeker et al,11 in 2011 for risk stratification focusing on the risk of recurrence, and they found that the size of > 5 cm and high mitotic rate were the only two factors significantly associated with recurrence after surgical resection. Our case shows a strong tendency to recur in view of the aforementioned risk, namely size and mitotic rate, hence adjuvant radiotherapy was provided, and a vigorous follow-up is required to ensure complete remission.
Conclusion
PEComas are a rare entity with variable presentation and tumor behavior. It is important to consider rarities when confronted with an atypical skin lesion. A multidisciplinary preoperative evaluation should include information obtained by fine-needle or core biopsy so that the appropriate surgical approach can be planned.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from the patient.
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this case report.
references
- 1. Charli-Joseph Y, Saggini A, Vemula S, Weier J, Mirza S, LeBoit PE. Primary cutaneous perivascular epithelioid cell tumor: a clinicopathological and molecular reappraisal. J Am Acad Dermatol 2014 Dec;71(6):1127-1136.
- 2. Llamas-Velasco M, Requena L, Mentzel T. Cutaneous perivascular epithelioid cell tumors: a review on an infrequent neoplasm. World J Methodol 2016 Mar;6(1):87-92.
- 3. Girardi FM, Nunes AB, Hauth LA. Malignant subcutaneous pecoma on the Cheek. An Bras Dermatol 2018;93(6):934-935.
- 4. Shon W, Kim J, Sukov W, Reith J. Malignant TFE3-rearranged perivascular epithelioid cell neoplasm (PEComa) presenting as a subcutaneous mass. Br J Dermatol 2016 Mar;174(3):617-620.
- 5. Stuart LN, Tipton RG, DeWall MR, Parker DC, Stelton CD, Morrison AO, et al. Primary cutaneous perivascular epithelioid cell tumor (PEComa): five new cases and review of the literature. J Cutan Pathol 2017 Aug;44(8):713-721.
- 6. Walsh SN, Sangüeza OP. PEComas: a review with emphasis on cutaneous lesions. Semin Diagn Pathol 2009 Aug;26(3):123-130.
- 7. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 2005 Dec;29(12):1558-1575.
- 8. Calder KB, Schlauder S, Morgan MB. Malignant perivascular epithelioid cell tumor (‘PEComa’): a case report and literature review of cutaneous/subcutaneous presentations. J Cutan Pathol 2008 May;35(5):499-503.
- 9. Kapoor A, Beniwal S, Singhal MK, Kumar N, Kumar V, Kumar HS. HMB-45 negative multifocal malignant perivascular epithelioid cell tumor of the soft tissue responding to sirolimus: first case report from India. J Cancer Res Ther 2015 Oct-Dec;11(4):1036.
- 10. Greveling K, Winnepenninckx VJ, Nagtzaam IF, Lacko M, Tuinder SM, de Jong JM, et al. Malignant perivascular epithelioid cell tumor: a case report of a cutaneous tumor on the cheek of a male patient. J Am Acad Dermatol 2013 Nov;69(5):e262-e264.
- 11. Bleeker JS, Quevedo JF, Folpe AL. “Malignant” perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma 2012;2012:541626.