Breast cancer is the most common cancer in women worldwide and a leading cause of death.1 However, due to mammogram screening, many cases with asymptomatic lesions are detected early, and ultimately the prognosis is much better.2 Nowadays, innumerable studies are looking for more histological and molecular features in the tumors that may reduce overtreatment and better predict prognosis.2
Recently, studies are focusing on the stroma surrounding the malignant cells in breast cancers, which provides the support and better growth environment for the tumor cells. The striking component is the accumulation of a large amount of elastin fibers in and around the tumor, a status known as elastosis.2 Elastic fibers are usually seen in hematoxylin and eosin (H&E) stained sections; however, quantifying elastosis requires elastin van Gieson (EVG) stain as a gold standard.1 The amount of these elastic fibers differs according to the nature of the lesion; it increases in benign breast diseases with the increment in the number of hyperplastic cells, and the amount is much more in the malignant lesions.1
Due to this relation between elastosis and breast cancer, some authors consider the presence of large aggregates of elastin in breast tissue as an indication of cancer.3 The first study of elastosis in breast cancer decades ago found that a large amount of elastin in breast lesions is associated with better prognosis and improved survival.4 Studies have also found an association between elastosis, estrogen receptor (ER), and progesterone receptor (PR), and better response to hormonal therapy.3,5 Subsequently, limited studies have addressed the prognostic value of elastosis in primary breast cancer.
For example, stromal elastosis is associated with good prognostic markers, including ER positivity, HER2 negativity, and lower Ki-67.2 They found that a high amount of elastosis is seen in morphologically highly differentiated tumors (low histological grade) compared to poorly differentiated tumors (high histological grade).2 Studies that looked at the relationship between elastosis and the two steroid receptors (ER and PR) observed a large amount of elastin associated with intense estrogen positivity. However, this relation is noticed to a lesser degree with PR.3,6 On the other hand, other studies found that the amount and the extent of elastosis in the stroma within the tumor shown to have little value in recurrence-free survival or prognosis.3,5,6
To date, no local data is available describing the trend of elastosis among Omani patients. As stated above, limited literature addressed the prognostic value of elastosis in breast cancer patients, which is still controversial and needs further exploration. Furthermore, there is no literature addressing the diagnostic accuracy of the H&E stain method in quantifying elastosis.
Our study aims to describe the occurrence of elastosis in invasive breast carcinoma among Omani female patients using semi-quantitative methods (H&E and EVG staining). In addition, we sought to investigate further the relationship of elastosis with the prognosis and prognostic markers, including ER positivity, PR, HER2/neu receptor, tumor grade, and Ki-67 index. Furthermore, we evaluated the diagnostic accuracy of the H&E stain method in quantifying elastosis compared to EVG.
Methods
In this retrospective study, data of female patients diagnosed with breast cancer who underwent biopsy or resection surgery in the Armed Forces Hospital in Muscat, Oman, between 2009 and 2019 were retrieved from the electronic hospital records. Patients with post neoadjuvant chemotherapy resection were excluded from the study.
Data retrieved from the medical records included age, menopausal status, tumor type and grade, ER, PR, Ki-67, and HER2/neu status. Follow-up data including clinical remission, evidence of metastasis, death, or lost to follow-up was also obtained. The age was further subcategorized into < 30, 30–40, 40–50, 50–60, and > 60 years. Tumors were classified as ductal, lobular, and others. Tumor grading was done according to the Nottingham criteria. Ki-67 was also categorized as ≤ 20% and ≥ 20%.
H&E stained slides of all cases were retrieved, and an additional special stain, EVG, to detect elastosis was done for all cases and considered the gold standard. Both slides (H&E and EVG stained slides) were examined and evaluated under microscopy for the amount of elastosis.
Table 1: Grading system of elastosis using hematoxylin and eosin and elastin van Gieson stains.
|
Absent |
No elastosis is detected |
|
Grade 1 |
Small deposits (single elastin fibrils or a thin rim of elastosis around ducts) were present |
|
Grade 2 |
Thicker zones of elastosis were found |
Elastosis was graded according to a previously published grading system,2 using a semi-quantitative manner by two pathologists independently. The grading system is illustrated in Table 1. The revealed grades were further subcategorized into low elastosis (absent, grade 1, and grade 2) and high elastosis (grade 3). Elastosis grading was done blindly of other features.
The collected information was entered using Epi-data program and then transferred to SPSS Statistics (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) for analysis. The chi-square test assessed associations between different categorical variables. In addition, univariate survival analyses of time to death due to breast cancer (disease-specific survival) was performed using the Kaplan–Meier survival curves with log-rank test for comparisons. Two-sided p-values of < 0.050 were considered statistically significant. This study was ethically approved by the Histopathology Department of the Armed Forces Hospital.
Results
A total of 80 cases, including 40 biopsies and 40 resection specimens, were included. The patients’ age ranged from 27–83 years, with a mean age of 51.7±13.3 years with a median of 52.5 years (minimum of 27 and maximum of 80). Among the total sample, 80.0% (n = 64) were diagnosed with invasive ductal carcinoma (NOS), while 12.5% (n = 10) of cases were diagnosed with infiltrating lobular carcinoma. The remaining 7.5% (n = 6) cases were tubular, medullary, and mucinous carcinomas. Over half (52.5%) of our sample were postmenopausal cases. More than half of the cases were graded as Nottingham grade 2 (n = 41, 51.3%), while grade 1 and grade 3 constituted 23.8% (n = 19) and 25.0% (n = 20), respectively. Throughout the study period, the patients’ follow-up ranged from 0.03 (3 months)–10 years with a mean of 43.0±28.3 months and a median of 33.0 months, a minimum of 3 and a maximum of 120 months.
Using EVG, absent elastosis, grade 1, grade 2, and grade 3 were observed in 12.5%, 37.5%, 30.0%, and 20.0%, respectively. Elastosis grading on H&E conducted by the two pathologists independently revealed strong interobserver agreement (K = 0.858). Eleven out of 13 cases were agreed on high elastosis (84.6%), and 66 out of 67 (98.5%) cases were agreed to be low elastosis by the two assessors.
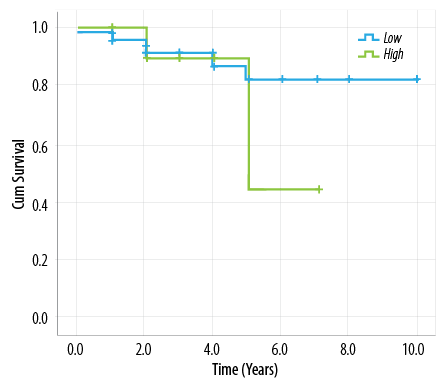
Figure 1: Survival among high and low elastosis groups.
Table 2: 2 × 2 table of hematoxylin and eosin (H&E) compared to elastin van Gieson stain (EVG) methods in quantifying elastosis.
|
Low elastosis |
62 |
5 |
67 |
|
High elastosis |
2 |
11 |
13 |
A statistically significant relationship between high elastosis and ER positivity was observed (p = 0.015). In this regard, 60.9% of low elastosis cases were ER positive compared to 93.6% of high elastosis cases. Among low elastosis cases, 50.0% were HER2 negative compared to 81.2% among high elastosis. This difference was statistically significant (p = 0.045), indicating the strong relationship between high elastosis and HER2 negativity. On the other hand, no statistically significant relationship between elastosis and other entities, including menopausal status, tumor type and grade, PR, and Ki-67 was observed.
The overall five-year survival in our patients was 77.9%. High elastosis patients did not show any advantage for the overall survival rate than low elastosis patients (p = 0.500). Figure 1 compares the survival curves among high and low elastosis groups, which shows almost similar survival until the fifth year of follow-up. In addition, there was no difference between the two groups in relation to clinical remission (62.5% vs. 68.8% in low and high elastosis groups respectively, p = 0.500).
Concerning the diagnostic accuracy of H&E stain in quantifying elastosis, Table 2 illustrates the 2 × 2 table of H&E compared to EVG stain methods in quantifying elastosis. The sensitivity and specificity of H&E stained method compared to EVG stained method (the gold standard) were 68.8% and 96.9%, respectively. The positive predictive value was 84.6%, negative predictive value was 92.5%, positive likelihood ratio was 22.0, and the negative likelihood ratio was 0.3.
Discussion
This is the first study addressing the trend of elastosis among breast cancer patients in Oman, and the diagnostic accuracy of H&E stain in quantifying elastosis worldwide. This study showed the common occurrence of elastosis among local patients and emphasized the significant association between elastosis in breast cancer and estrogen positivity. In addition, this study showed good diagnostic value for H&E stain in quantifying elastosis.
The amount of elastic fibers in breast tissue and especially in malignant cases is an indicator of a good prognosis in such patients.7,8 This has been evaluated in previous studies, which showed different results.1–3 For example, in a study by Rasmussen et al,3 the trend of elastic tissue content among 171 primary breast carcinomas was different compared to our figures. They observed that 35% of cases showed absent or grade 1 elastosis, 42% of cases presented with grade 2 elastosis, and 22% had massive elastosis (grade 3). This variation in the occurrence of elastosis in different populations can be explained by differences in ethnicity and genetics, and differences in the occurrence of various subtypes of breast cancer studied the relatively low number of ducts in the circumscribed and expansible tumors that displace these ducts.7 In addition, it differs depending on the degree of anaplasia, being more in the well-differentiated malignant lesions compared to the poorly-differentiated malignant lesions.3 Moreover, elastosis occurrence differs significantly in cases not yet treated compared to cases examined after treatment.2
Regarding the relationship between elastosis and steroid receptors, Rasmussen et al,3 observed that a large amount of elastin is associated with intense estrogen positivity. However, this relationship is noticed in a lesser degree with the PR.3,9 Similar results were observed in different studies as well as our study.1,6,8 On the other hand, few studies observed that even ER and PR negative breast cancers are positive for elastosis. For example, 68.75% of ER and PR negative cases were positive for elastosis.7 This was attributed to confounding factors, especially the tumor type (triple-negative tumors).1,7
Many studies have observed the association between HER2 and Ki-67 and elastosis.1–3 In this regard, Chen et al,2 found that stromal elastosis is almost always associated with factors that give a good prognosis, including HER2 negativity and low Ki-67. We observed similar results between elastosis and HER2 negativity; however, our results are conflicting with other studies for Ki-67, as we observed no association between elastosis and Ki-67.
The value of elastosis in survival has been investigated in other studies, and the results have been contradictory. Some studies found that elastosis in breast cancer is associated with better prognosis and prolonged survival. This may be explained as tumors with no elastosis were more aggressive and associated with tumor necrosis, lymph node metastasis, and vascular invasion.1,2 The results of one study suggested that elastosis is probably not a favorable microenvironment for tumor growth and spread.2
The amount and the extent of elastosis in the stroma within the tumor in our study show to have little value in disease-specific survival or prognosis. This is consistent with other studies, including a study that showed that there was better survival in patients with extensive elastosis when compared with those who had none, but that difference was so small as to be of little consequence to the overall duration of survival.5,10 On the other hand, Glaubitz et al,3 found a negative effect of elastosis on survival.
In relation to the diagnostic accuracy of H&E stain in quantifying elastosis, H&E is considered the primary stain and the most commonly used staining method for histology slides. Pathologists by far prefer it for viewing different cellular and various tissue structural details. It contains the two dyes H&E. Hematoxylin gives the acidic (or basophilic) structures with a purplish blue color while eosin stains basic (or acidophilic) structures with red or pink. Elastic fibers can be recognized in standard H&E slides as a deposit of grey fibrillary material.2 In contrast, EVG gives these fibers black color, which explains a higher sensitivity than the visual recognition of the grey material in H&E slides.2 However, due to the high sensitivity of H&E, only a small proportion of H&E slides with absent elastosis were in fact grade 1 by EVG, and likewise for H&E grade 1 being grade 2 in EVG. However, there was almost complete concordance between H&E and EVG in quantifying grade 3 cases.
Conclusion
This study is the first study addressing elastosis among Omani breast cancer patients and addressing the accuracy of H&E stain in quantifying elastosis. Elastosis occurrence varies in different breast cancer populations. We emphasize the strong relationship between high elastosis and estrogen positivity and negative HER2/neu receptor among breast cancer patients. Hence the presence of elastosis can be considered a surrogate marker for estrogen positivity in the initial screening procedure. In addition, H&E stain is regarded as an accurate method for quantifying elastosis compared to the EVG staining method.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Gupta D, Gupta V, Marwah N, Gill M, Gupta S, Gupta G, et al. Correlation of hormone receptor expression with histologic parameters in benign and malignant breast tumors. Iran J Pathol 2015;10(1):23-34.
- 2. Chen Y, Klingen TA, Wik E, Aas H, Vigeland E, Liestøl K, et al. Breast cancer stromal elastosis is associated with mammography screening detection, low Ki67 expression and favourable prognosis in a population-based study. Diagn Pathol 2014 Dec;9(1):230.
- 3. Rasmussen B, Pedersen V, Thorpe SM, Rose C. Elastosis in relation to prognosis in primary breast carcinoma. American Association for Cancer research 1985;45(3):1428-1430.
- 4. Shivas AA, Douglas JG. The prognostic significance of elastosis in breast carcinoma. J R Coll Surg Edinb 1972;17:315-320.
- 5. Davies JD, Barnard K. Prognostic value of measurement of elastosis in breast carcinoma. J Clin Pathol 1982 Feb;35(2):245-245.
- 6. Jacquemier J, Lieutaud R, Martin PM. Relationship of stromal elastosis to steroid receptors in human breast carcinoma. Recent Results Cancer Res 1984;91:169-175.
- 7. Azzopardi JG, Laurini RN. Elastosis in breast cancer. Cancer 1974 Jan;33(1):174-183.
- 8. Masters JR, Millis RR, King RJ, Rubens RD. Elastosis and response to endocrine therapy in human breast cancer. Br J Cancer 1979 May;39(5):536-539.
- 9. Masters JR, Hawkins RA, Sangster K, Hawkins W, Smith II, Shivas AA, et al. Oestrogen receptors, cellularity, elastosis and menstrual status in human breast cancer. European Journal of Cancer (1965) 1978 Mar 1;14(3):303-307.
- 10. Carlomagno C, Perrone F, Lauria R, de Laurentiis M, Gallo C, Morabito A, et al. Prognostic significance of necrosis, elastosis, fibrosis and inflammatory cell reaction in operable breast cancer. Oncology 1995 Jul-Aug;52(4):272-277.