Globally, the number of people with diabetes has increased significantly in recent decades. A similar trend is documented in Malaysia. In 2011 the prevalence of diabetes was 15.2%, and it is projected to reach 21.6% in 2020, affecting approximately 4.5 million Malaysians aged 18 years and above.1 This trend is attributed to the increasing prevalence of overweight and obesity resulting from physical inactivity and high consumption of sugar and fatty food in both developed and developing countries.2,3
As the current epidemic of obesity and diabetes has increased, the prevalence of type 2 diabetes mellitus (T2DM) in women of childbearing age and the number of pregnant women with undiagnosed T2DM has also increased.4 Likewise, the number of gestational diabetes mellitus (GDM) cases has grown in recent decades.4 GDM is first diagnosed during the second or third trimester of pregnancy, and whether this is either a pre-existing type 1 diabetes or T2DM is unclear.5 There is a strong association between GDM and T2DM. Women with GDM have at least seven times higher risk of developing T2DM than those with normal pregnancies.6 In addition, untreated carbohydrate intolerance during pregnancy causes maternal morbidity and perinatal morbidity and mortality.7 In women with GDM, insulin resistance already exists before pregnancy, but it worsens during pregnancy. Insulin secretion is inadequate to compensate for the insulin resistance, leading to hyperglycemia, which is detected by routine glucose screening during pregnancy. Thus, chronic insulin resistance is a central component of the pathophysiology of women with GDM.8
Carbohydrate intolerance during pregnancy usually resolves after delivery. However, postpartum testing reveals that up to one-third of affected women have DM or impaired glucose tolerance.5,9 Glucose testing performed at six weeks postpartum may delay or prevent the onset of T2DM, which manifests soon after the postpartum period, through lifestyle modification, such as dietary changes, physical activity, weight management, and/or pharmacological intervention.9 However, most women with GDM are not screened for T2DM after delivery; thus, diabetes prevention and treatment opportunities are missed. A scheduled postpartum test is important to detect prediabetes and diabetes and allow prompt intervention to reduce the risk of diabetes or diabetes-related complications.10
Malaysia follows the recommended protocol of glucose testing at six weeks postpartum. During the first postpartum visit in primary health care clinics, all women with GDM are given an appointment for the test at six weeks postpartum. These primary health care facilities, which women attend for their antenatal check-ups, are easily accessible as they are situated near the women’s place of residence, approximately 5 km away in the urban areas and 3 km away in rural areas. The services offered in these health clinics include maternal and child health services, which provide antenatal and postnatal care to all mothers. Despite the availability and accessibility of these primary care services, there is no existing system that reminds women to undergo postpartum glucose testing.11,12
Given that early detection of glucose abnormalities postpartum can delay or prevent T2DM in women with glucose intolerance, postpartum glucose testing is important because it allows early detection of prediabetes and treatment of diabetes. Our study determined the current postpartum glucose testing rate among women with GDM. To our knowledge, this study is the first to determine the compliance rate of women with GDM for glucose testing at six weeks postpartum and the associated factors influencing the compliance with the test in the southern part of Peninsular Malaysia.
METHODS
This cross-sectional study was conducted in Johor Bahru District, the state capital city of Johor. The state’s geographical position being the southernmost city in Peninsular. Johor has the second largest population in Malaysia at 3 230 440 in 2010, increasing to 3 601 690 in 2016.13 The state’s ethnic composition include Malays (51.2%), Chinese (33.5%), Indian (10.7%), other ethnic groups (0.1%), and non-citizens (4.5%). Johor Bahru District has 16 government primary health care clinics, but only 13 offer maternal and child health services.
This study was conducted based on the medical records of postpartum women who had registered in primary health care clinics in Johor Bahru from January to June 2016 and had GDM in their most recent pregnancy. Those who had their postpartum follow-up in other districts or were unable to attend testing because of physical disability (e.g., being bed-ridden) were excluded. Since this study involved reviewing secondary data, records missing at least one of the required information were excluded. This study was approved by the Human Research Ethics Committee of the Universiti Sains Malaysia [USM/JEPeM/16120599] and the Medical Research and Ethics Committee of the Ministry of Health, Malaysia [NMRR-16-2352-33522(IIR)].
The sample size was estimated using the single proportion formula to determine the proportion of individuals who undergo postpartum glucose testing and using power and sample size calculation software to compare two independent proportions. From all the sample sizes determined, the biggest sample size was 345. A total of 516 postpartum women with GDM were registered from January to June 2016. Of these, 341 cases met the inclusion criteria, and the records of all these women were reviewed.
This study involved secondary data collection from primary health care clinics. A list of women with GDM was obtained. Sociodemographic, clinical, obstetric characteristics, and glucose screening results during pregnancy and after delivery were extracted from their maternal health records. The retrieved information were reviewed and recorded in the data collection form by a researcher.
The maternal health record was assigned during the women’s first antenatal check-up and maintained until six weeks after delivery. Information related to the pregnancy, delivery, and postpartum period was recorded. The record was prepared in two copies for each pregnancy; one copy was a home-based card, which belongs to the pregnant women, and the respective health clinic kept the other copy.
The record consists of three sets of information: the women’s sociodemographic, clinical, and healthcare data. The sociodemographic information collected in this study included the women’s age, ethnicity, marital status, educational level, and occupation. Educational level was classified into secondary and lower education or tertiary and higher education.
Clinical data included family history of diabetes, parity, and previous diagnosis of GDM. The women diagnosed with GDM in pregnancies before their most recent pregnancy were classified as having a prior diagnosis of GDM. Other data obtained include any underlying medical illness and gestational age at booking. Booking is defined as the first antenatal visit of the respondents to any clinic. It is classified according to gestational age at the time of booking, namely, early booking, which is done before 12 weeks of gestation, and late booking, which is done after 12 weeks gestation. In the maternal health record, information such as gestational weight gain, treatment with metformin or insulin, and glycated hemoglobin (HbA1c) level were recorded by the attending physicians at the health clinics. Other clinical data retrieved include mode of delivery, infant birth weight, and admission to a neonatal intensive care unit. These data were all recorded in the postnatal column found in the last pages of the maternal health record.
In addition, postpartum, home, and hospital follow-up visits were among the health care data obtained from the maternal health record. During each visit, the dates for subsequent follow-up visits in the clinic or hospital setting were recorded. Data were recorded in the maternal health record during each home visit by an attending nurse. Information regarding postpartum glucose testing compliance and the test results were recorded in the postnatal column of the maternal health record. The women were indicated to have undergone postpartum glucose testing if they were tested six weeks postpartum as scheduled on their appointment date. Moreover, the women were indicated to have abnormal glucose tolerance if they had diabetes, prediabetes, impaired glucose tolerance, impaired fasting glucose, or elevated glucose tolerance.14
All information were analyzed using SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Data were expressed as frequency and percentages for categorical variables. Simple and multiple logistic regression analyses were used to evaluate the factors associated with compliance with postpartum glucose testing. All variables were tested for their association with postpartum glucose testing. The dependent variable was postpartum glucose testing, which was either performed or not performed. In variable selection, the stepwise, forward and backward methods were used. In selecting a model, the three rules considered were statistical significance, parsimony, and biological plausibility. Any possible two-way interaction was checked in the model. The fitness of the model was tested using the Hosmer–Lemeshow goodness of fit test. The classification table and area under receiver operating characteristic curve were used to determine the fitness of the model. The final model was presented in terms of adjusted odds ratio (AOR), 95% confidence interval (CI), Wald statistics, and p-value. The significance level was set at p < 0.050.
Table 1: Sociodemographic and clinical characteristics of women with gestational diabetes mellitus (GDM) (n = 341).
|
Age, years |
31.7 ± 4.9 |
|
|
|
Ethnicity |
|
|
|
|
Malay |
|
213 |
62.5 |
|
Chinese |
|
70 |
20.5 |
|
Indian |
|
41 |
12.0 |
|
Others |
|
17 |
5.0 |
|
Marital status |
|
|
|
|
Married |
|
337 |
98.8 |
|
Single |
|
4 |
1.2 |
|
Education |
|
|
|
|
Tertiary and above |
|
13 |
3.8 |
|
Secondary and lower |
|
328 |
96.2 |
|
Parity |
|
|
|
|
Multiparous |
|
224 |
65.7 |
|
Primiparous |
|
117 |
34.3 |
|
Family history of diabetes |
|
|
|
|
No |
|
167 |
49.0 |
|
Yes |
|
174 |
51.0 |
|
Previous diagnosis of GDM |
|
|
|
|
No |
|
267 |
78.3 |
|
Yes |
|
74 |
21.7 |
|
Gestational age at booking, weeks |
|
|
|
|
≥ 12 |
|
170 |
49.9 |
|
< 12 |
|
171 |
50.1 |
|
Underlying medical illness |
|
|
|
|
No |
|
299 |
87.7 |
|
Yes |
|
42 |
12.3 |
|
Insulin usage |
|
|
|
|
No |
|
305 |
89.4 |
|
Yes |
|
36 |
10.6 |
|
Gestational weight gain |
|
|
|
|
Normal |
|
185 |
54.3 |
|
Excessive |
|
48 |
14.1 |
|
Poor |
|
108 |
31.7 |
|
Glycated hemoglobin (HbA1c) |
|
|
|
|
Abnormal (> 6.5 mmol/L) |
|
24 |
7.0 |
|
Normal (< 6.5 mmol/L) |
|
317 |
93.0 |
|
Mode of delivery |
|
|
|
|
Cesarian section |
|
89 |
26.1 |
|
Vaginal delivery |
|
252 |
73.9 |
|
Infant birth weight, g |
|
|
|
|
2500–3500 |
|
265 |
77.7 |
|
< 2500 |
|
24 |
7.0 |
SD: standard deviation.
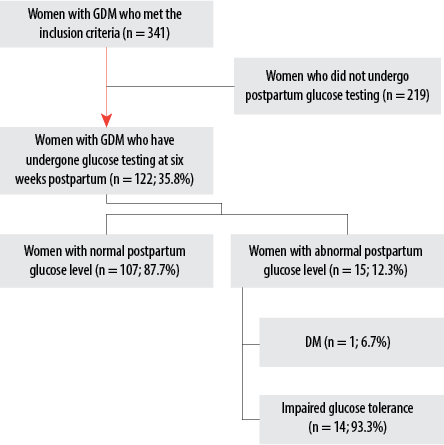
Figure 1: Flow chart showing the prevalence of abnormal glucose tolerance among postpartum women with gestational diabetes mellitus (GDM) who have undergone glucose testing.
RESULTS
A total of 341 postpartum women with GDM were included in this study. Their age ranged between 18 and 48 years with a mean (±SD) of 31.7±4.9 years. Majority of subjects were Malays (n = 213; 62.5%), married (n = 337; 98.8%), and had secondary and lower education (n = 328; 96.2%). Table 1 shows the sociodemographic and clinical characteristics of the subjects.
The proportion of women who showed up in primary healthcare clinics for glucose testing at six weeks postpartum was 35.8%. The remaining 219 (64.2%) did not show up during their appointments, which their attending healthcare providers set during their routine follow-up within the immediate postpartum period. Among those who underwent postpartum glucose testing, 107 (87.7%) were normoglycemic, and 15 (12.3%) had abnormal glucose tolerance at six weeks postpartum. Out of the 15 cases with abnormal glucose tolerance, one patient (6.7%) was diagnosed with DM, and 14 (93.3%) were diagnosed with impaired glucose tolerance. Figure 1 shows the prevalence of abnormal glucose tolerance among postpartum women with GDM who have undergone glucose testing.
Simple logistic regression analysis was conducted to determine the factors associated with postpartum glucose testing among women with GDM. No significant association between sociodemographic factors and glucose testing at six weeks postpartum was found among women with GDM in Johor Bahru. The age, ethnicity, educational level, and employment status of the women did not significantly influence their behavior toward postpartum glucose testing. By contrast, the significant variables in this univariable analysis were family history of diabetes (p = 0.028), previous diagnosis of GDM (p = 0.020), and HbA1c level (p = 0.021) [Table 2].
Table 2: Factors associated with six weeks postpartum glucose testing using simple logistic regression
analysis (n = 341).
|
Age, years |
31.7 ± 5.1 |
|
31.6 ± 4.7 |
|
1.00 (0.96–1.05) |
0.00 (1) |
0.949 |
|
Parity |
|
|
|
|
|
|
|
|
Multiparous |
|
82 (67.2) |
|
142 (64.8) |
1 |
|
|
|
Primiparous |
|
40 (32.8) |
|
77 (35.2) |
1.11 (0.69–1.78) |
0.01 (1) |
0.658 |
|
Ethnicity |
|
Malay |
|
79 (64.8) |
|
134 (61.2) |
1 |
|
|
|
Chinese |
|
25 (20.5) |
|
45 (20.5) |
0.94 (0.54–1.65) |
0.04 (1) |
0.836 |
|
Indian |
|
12 (9.8) |
|
29 (13.2) |
0.70 (0.34–1.45) |
0.91 (1) |
0.341 |
|
Others |
|
6 (4.9) |
|
11 (5.0) |
0.93 (0.33–2.59) |
0.02 (1) |
0.883 |
|
Education level |
|
No higher education |
|
115 (94.3) |
|
213 (97.3) |
1 |
|
|
|
Higher education |
|
7 (5.7) |
|
6 (2.7) |
2.16 (0.71–6.58) |
1.84 (1) |
0.175 |
|
Employment status |
|
Unemployed |
|
60 (49.2) |
|
121 (55.3) |
1 |
|
|
|
Employed |
|
62 (50.8) |
|
98 (44.7) |
1.28 (0.82–1.99) |
1.16 (1) |
0.282 |
|
Family history of diabetes |
|
No |
|
50 (41.0) |
|
117 (53.4) |
1 |
|
|
|
Yes |
|
72 (59.0) |
|
102 (46.6) |
1.65 (1.06–2.59) |
4.82 (1) |
0.028 |
|
Previous diagnosis of
gestational diabetes mellitus |
|
No |
|
87 (71.3) |
|
180 (82.2) |
1 |
|
|
|
Yes |
|
35 (28.7) |
|
39 (17.8) |
1.86 (1.10–3.13) |
5.37 (1) |
0.020 |
|
Gestational age at
booking, weeks |
|
≥ 12 |
|
57 (46.7) |
|
113 (51.6) |
1 |
|
|
|
< 12 |
|
65 (53.3) |
|
106 (48.4) |
0.82 (0.53–1.28) |
0.75 (1) |
0.388 |
|
Underlying medical illness |
|
No |
|
102 (83.6) |
|
197 (90.0) |
1 |
|
|
|
Yes |
|
20 (16.4) |
|
22 (10.0) |
0.57 (0.29–1.09) |
2.87 (1) |
0.090 |
|
Insulin usage |
|
|
|
|
|
|
|
|
No |
|
108 (88.5) |
|
197 (90.0) |
1 |
|
|
|
Yes |
|
14 (11.5) |
|
22 (10.0) |
1.16 (0.57–2.36) |
0.17 (1) |
0.681 |
|
Gestational weight gain |
|
Normal |
|
69 (56.6) |
|
116 (53.0) |
1 |
|
|
|
Excessive |
|
20 (16.4) |
|
28 (12.8) |
1.20 (0.63–2.29) |
0.31 (1) |
0.579 |
|
Poor |
|
33 (27.0) |
|
75 (34.2) |
0.74 (0.45,1.23) |
1.36 (1) |
0.243 |
|
Glycated hemoglobin (HbA1c) |
|
Abnormal |
|
14 (11.5) |
|
10 (4.6) |
1 |
|
|
|
Normal |
|
108 (88.5) |
|
209 (95.4) |
2.71 (1.17–6.30) |
5.36 (1) |
0.021 |
|
Mode of delivery |
|
Caesarian section |
|
33 (27.0) |
|
56 (25.6) |
1 |
|
|
|
Vaginal delivery |
|
89 (73.0) |
|
163 (74.4) |
1.08 (0.65–1.78) |
0.09 (1) |
0.766 |
|
Infant birth weight, g |
|
2500–3500 |
|
91 (74.6) |
|
174 (79.5) |
1 |
|
|
|
< 2500 |
|
9 (7.0) |
|
15 (6.8) |
1.15 (0.48–2.72) |
0.10 (1) |
0.755 |
|
> 3500 |
|
22 (18.4) |
|
30 (13.7) |
1.42 (0.77–2.57) |
1.20 (1) |
0.274 |
|
Hospital follow-up |
|
No |
|
93 (76.2) |
|
183 (83.6) |
1 |
|
|
|
Yes |
|
29 (23.8) |
|
36 (16.4) |
0.63 (0.36–1.09) |
2.70 (1) |
0.100 |
|
NICU admission |
|
No |
|
105 (86.1) |
|
193 (88.1) |
1 |
|
|
SD: standard deviation; OR: odds ratio; CI: confidence interval; NICU: neonatal intensive care unit.
Multiple logistic regression analysis was used to identify the associated factors after controlling for all other variables. Educational level, family history of diabetes, previous diagnosis of GDM, presence of underlying medical illness, hospital follow-up, and HbA1c level were the variables with a p-value of < 0.050; thus, they were selected for multiple logistic regression analysis. From this analysis, the significant factors associated with postpartum glucose testing at six weeks among women with GDM when other variables were controlled were previous diagnosis of GDM (AOR = 1.76; 95% CI: 1.04–2.99; p = 0.036) and normal HbA1c level (AOR = 2.49; 95% CI: 1.06–5.86; p = 0.036) [Table 3].
Table 3: Factors associated with six-week postpartum glucose testing using multiple logistic regression
(n = 341).
|
Previous diagnosis of
gestational diabetes mellitus |
|
No |
|
1 |
|
|
|
Yes |
0.567 |
1.76 (1.04–2.99) |
4.40 (1) |
0.036 |
|
Glycated hemoglobin |
|
|
|
|
|
Abnormal |
|
1 |
|
|
B: regression coefficient; OR: odds ratio; CI: confidence interval.
DISCUSSION
This study examined the compliance with postpartum glucose testing for diabetes detection among women diagnosed with GDM during their most recent pregnancy. These women were registered in government primary health care clinics in Johor Bahru from January to June 2016. The postpartum glucose screening rate among this cohort of vulnerable women was low at 35.8%, consistent with previous results wherein postpartum screening rates ranged from 19–67%.14–17
There are several potential explanations for the low postpartum testing rates reported in this study. The results indicate that postpartum follow-up of women with GDM was insufficiently incorporated into the primary care system. Women with GDM have limited knowledge about their true risk of T2DM after delivery. They do not practice other positive preventive lifestyle changes, such as increasing the frequency of exercise even if they perceive that they are at high risk for T2DM. For postpartum testing to be facilitated, the risk of T2DM must be discussed during routine antenatal care, and offering of continuing education program during the postpartum period is essential to increase risk perception among women with GDM.18 However, this measure might be influenced by lack of approved protocols, insufficient or unclear communication from the attending physician in secondary care, and lack of sufficient reminder and tracking systems in primary care practices.19 Postpartum glucose testing rates after GDM may be increased by incorporating alerts into electronic medical records to ensure that postpartum glucose testing orders are automatically generated or are physician-ordered. An automated live or recorded telephone or email message could also be used to remind women to go for the test after it was ordered.9 In previous studies, the use of a reminder system involving a short messaging system to ensure that mothers show up for postpartum tests has resulted in high postpartum glucose testing rates.20,21 However, in Malaysia, a systematic reminder system that reminds women to show up for postpartum glucose testing does not exist. Although women with GDM who show up for their first postpartum visit to health clinics were given an appointment date for the test at six weeks postpartum, no further follow-up or reminder was given.
In this study, women with normal HbA1c levels during pregnancy have approximately 2.5 times higher odds of showing up for postpartum glucose testing at six weeks postpartum than those with abnormal results.
Studies have examined the association between HbA1c level and compliance for postpartum glucose testing, but all of these studies have found no associations between the two.14,22,23 The reason behind the current findings, particularly for women with GDM, is unclear. However, the current finding may be a reflection of a ‘healthy cohort’ effect, in which individuals with normal HbA1c levels are more health-conscious and likely to seek treatment or to show up for a check-up.15
Moreover, this study found that previous diagnosis of GDM significantly influenced the compliance for postpartum glucose testing. The women diagnosed with GDM in their pregnancies before the most recent one have approximately two times higher odds of attending postpartum glucose testing. Women diagnosed with GDM in their previous pregnancies may have already gained some basic knowledge about the disease and its future implication; thus, they are more likely to return for the screening test.21 By contrast, those with no prior diagnosis of GDM had a lower awareness score than those with current or former GDM, given that they had not received any information or health advice and education on the subject. Nevertheless, many of them had already presented several risk factors for GDM and T2DM, including high body mass index, family history of diabetes, non-white ethnicity, and smoking.24
A study in the USA has found that women who failed to return for postpartum glucose testing are more likely to have a history of GDM in their previous pregnancies compared with women who returned.25 However, other studies conducted in the USA, Australia, Brazil, and Canada to investigate the influence of a history of GDM in previous pregnancies on postpartum testing did not find statistically significant associations between the two.6,16,23,26,27 Meanwhile, other studies have concluded that the knowledge and awareness of women with GDM on the importance of postpartum glucose testing may be limited.19 Thus, although they have been diagnosed with GDM in previous pregnancies and despite understanding the association between GDM and development of T2DM, those women did not perceive themselves to be at risk of future diabetes.28
Some limitations should be kept in mind when interpreting our findings. As our study findings were based on maternal health record, other factors were missed, such as patients’ perception and health belief on the risk of developing diabetes, difficulty in assessing health services, and quality of health services. Further studies are needed to explore the other possible factors contributing to compliance with postpartum glucose testing.
Our study is the first to report the rate of compliance and the factors associated with compliance with postpartum glucose testing among women with GDM in primary health care in Johor Bahru, Malaysia. Despite the appointments given during the early postpartum period, many of these women failed to comply. The proportion of women with GDM who showed up for postpartum glucose testing remained low, and improvements are needed. The detection rate of abnormal glucose status among those who showed up for the test was more or less similar to that reported in other studies. The significant factors associated with compliance to postpartum glucose testing include a previous diagnosis of GDM and a normal HbA1c level during pregnancy. Strategies must be reinforced to increase the compliance of more women with GDM for the test.
Conclusion
The postpartum glucose testing rate of women with GDM in our population was low. Women with normal HbA1c levels during pregnancy and those with a previous diagnosis of GDM were more likely to attend postpartum glucose testing at six weeks. Strategies to enhance compliance for postpartum glucose testing should be strengthened. Educating all women with GDM is essential, with special emphasis to those having poor glycemic control during pregnancy.
Acknowledgements
The authors acknowledge Universiti Sains Malaysia and the Ministry of Health Malaysia for approving the conduct of this study. The authors also acknowledge the invaluable support from the Johor Bahru District Health Office staff in completing this study.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Mustapha F, Omar Z, Mihat O, Md Noh K, Hassan N, Abu Bakar R, et al. Addressing non-communicable diseases in Malaysia: an integrative process of systems and community. BMC Public Health 2014;14(Suppl 2):S4.
- 2. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care 2015 Apr-Jun;4(2):187-192.
- 3. Macdonald IA. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur J Nutr 2016 Nov;55(Suppl 2):17-23.
- 4. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016 Jan;16(1):7.
- 5. American Diabetes Association. 2. Classification and diagnosis of diabetes. diabetes care 2017 Jan;40(Suppl 1):S11-S24.
- 6. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009 May;373(9677):1773-1779.
- 7. Gasim T. Gestational diabetes mellitus: maternal and perinatal outcomes in 220 saudi women. Oman Med J 2012 Mar;27(2):140-144.
- 8. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007 Jul;30(Suppl 2):S112-S119.
- 9. Lawrence JM, Black MH, Hsu JW, Chen W, Sacks DA. Prevalence and timing of postpartum glucose testing and sustained glucose dysregulation after gestational diabetes mellitus. Diabetes Care 2010 Mar;33(3):569-576.
- 10. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, et al; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008 Dec;93(12):4774-4779.
- 11. Malaysia Ministry of Health. Perinatal care manual standard operating procedure. 3rd ed. Family Health Development Division. Putrajaya: Malaysia Ministry of Health. 2013. p.49.
- 12. Thomas S, Beh L, Nordin RB. Health care delivery in Malaysia: changes, challenges and champions. J Public Health Afr 2011 Sep;2(2):e23.
- 13. Malaysia Department of Statistics. Demographic and population: total population by ethnic group, local authority and state, Malaysia. 2016 [cited 2016 March 4]. Available from: https://newss.statistics.gov.my/newss-portalx/ep/epLogin.seam.
- 14. Jindal R, Siddiqui MA, Gupta N, Wangnoo SK. Prevalence of glucose intolerance at 6 weeks postpartum in Indian women with gestational diabetes mellitus. Diabetes Metab Syndr 2015 Jul-Sep;9(3):143-146.
- 15. Cho GJ, An JJ, Choi SJ, Oh SY, Kwon HS, Hong SC, et al. Postpartum glucose testing rates following gestational diabetes mellitus and factors affecting testing non-compliance from four tertiary centers in Korea. J Korean Med Sci 2015 Dec;30(12):1841-1846.
- 16. Korpi-Hyövälti E, Laaksonen DE, Schwab U, Heinonen S, Niskanen L. How can we increase postpartum glucose screening in women at high risk for gestational diabetes mellitus? Int J Endocrinol 2012;2012:519267.
- 17. Tovar A, Chasan-Taber L, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis 2011 Nov;8(6):A124.
- 18. Keely E. An opportunity not to be missed–how do we improve postpartum screening rates for women with gestational diabetes? Diabetes Metab Res Rev 2012 May;28(4):312-316.
- 19. Koning SH, Lutgers HL, Hoogenberg K, Tromepert CA, van den Berg PP, Wolffenbuttel BH. Postpartum glucose follow-up and lifestyle management after gestational diabetes mellitus: general practitioner and patient perspectives. J Diabetes Metab Disord 2016 Dec;15(1):56.
- 20. Ko JY, Dietz PM, Conrey EJ, Rodgers L, Shellhaas C, Farr SL, et al. Gestational diabetes mellitus and postpartum care practices of nurse-midwives. J Midwifery Womens Health 2013 Jan-Feb;58(1):33-40.
- 21. Mohd Suan MA. Return for postpartum oral glucose tolerance test following gestational diabetes mellitus. Asia Pac J Public Health 2015 Sep;27(6):601-609.
- 22. Kwong S, Mitchell RS, Senior PA, Chik CL. Postpartum diabetes screening: adherence rate and the performance of fasting plasma glucose versus oral glucose tolerance test. Diabetes Care 2009 Dec;32(12):2242-2244.
- 23. Weinert LS, Mastella LS, Oppermann ML, Silveiro SP, Guimarães LS, Reichelt AJ. Postpartum glucose tolerance status 6 to 12 weeks after gestational diabetes mellitus: a Brazilian cohort. Arq Bras Endocrinol Metabol 2014 Mar;58(2):197-204.
- 24. Rivas A, Landon M, Gaillard T, Schuster D, Osei K. Awareness of risk factors for type 2 diabetes in women with current and former gestational diabetes mellitus (GDM): implications for future primary diabetes prevention. Diabetes Metab Syndr 2010 Apr-Jun;4(2):89-94.
- 25. Carson MP, Frank MI, Keely E. Original research: postpartum testing rates among women with a history of gestational diabetes–systematic review. Prim Care Diabetes 2013 Oct;7(3):177-186.
- 26. Capula C, Chiefari E, Vero A, Iiritano S, Arcidiacono B, Puccio L, et al. Predictors of postpartum glucose tolerance testing in italian women with gestational diabetes mellitus. ISRN Endocrinol 2013 Jul;2013:182505.
- 27. Shea AK, Shah BR, Clark HD, Malcolm J, Walker M, Karovitch A, et al. The effectiveness of implementing a reminder system into routine clinical practice: does it increase postpartum screening in women with gestational diabetes? Chronic Dis Can 2011 Mar;31(2):58-64.
- 28. Parsons J, Ismail K, Amiel S, Forbes A. Perceptions among women with gestational diabetes. Qual Health Res 2014 Apr;24(4):575-585.