While the prevalence of HIV infection in Oman continues to be < 1%,1 the prevalence of pediatric immunodeficiency (PID) is 7.0/100 000 with an estimated incidence of 5.0/100 000. These rates are higher in Oman than in Western populations, possibly reflecting a higher degree of consanguinity. Severe combined immunodeficiency (SCID) is the third most common type of PID (17.8%), following phagocytic and antibody disorders, whereas chronic diarrhea as the clinical presentation of PID is the fourth most common (10.7%) symptom, following pneumonia, deep abscesses, and Bacillus Calmette-Guérin (osis).2 Although no local data are available, intestinal cryptosporidiosis in children with PID is rare.
The incidence of cryptosporidiosis in Omani children is 1.9%. Cryptosporidium is the third most common protozoal organism identified in stool samples in children with intestinal symptoms.3 This parasite is increasingly recognized as an important zoonotic, food-, and waterborne enteric pathogen causing diarrheal illness in children in developing countries.4–7 Oocysts are transmitted fecal-orally, can resist routine chlorination and ozonation of water sources,8 and are excreted in the stool of an infected host. Although numerous Cryptosporidium species have been identified, humans are most frequently infected with Cryptosporidium hominis and Cryptosporidium parvum.6 Infection is usually self-limiting or asymptomatic in immunocompetent hosts but chronic and debilitating in immunocompromised children, especially those with profound T-cell lymphopenia and poor T-cell function.4–10 In children with immunodeficiency, the parasites infect and develop within the microvillus layer of small intestinal epithelial cells. Chronic infection is associated with villous atrophy, crypt hyperplasia, and secondary leucocytic infiltration in the lamina propria.7 In addition to chronic and considerable diarrhea, growth stunting, biliary tract disease, pancreatitis, and respiratory tract disease may occur.11 Treatment with antiprotozoal agents is usually ineffective in the context of immunodeficiency since they exhibit parasitistatic rather than parasiticidal activity.12
This report presents the description of the first Omani infant diagnosed with disseminated cryptosporidiosis in the context of SCID. The diagnostic and management challenges are highlighted along with a review of the literature.
Case report
A six-month-old girl was born to consanguineous parents at 37 weeks gestation and diagnosed with interleukin-7 receptor alpha deficiency, a monogenic cause of SCID. In the first few weeks of life, she was introduced to oral mashed meals using unboiled tap water derived from local wells (a culturally acceptable practice). She was well until 40 days of life when she presented with profuse watery, non-bloody diarrhea with vomiting. Upon admission, fever, cough, poor growth, and severe dehydration were observed. She exhibited severe wasting and a failure to thrive (weight, height, and head circumference were all below the third percentile). Preliminary investigations revealed electrolyte imbalance with metabolic acidosis, bilateral infiltrates on chest X-ray, and hepatomegaly and gallbladder wall thickening with debris on abdominal ultrasound. Computed tomography of the chest revealed non-specific bilateral ground-glass opacities, potentially related to a partially treated or ongoing pneumonia. The microbiological workup included analysis of bronchoalveolar lavage (BAL) and colonic tissue culture, revealing polymicrobial infection. Virus polymerase chain reaction (PCR), Mycobacterium PCR and cultures, and Pneumocystis PCR were negative [Table 1]. Stool examination using Ziehl–Neelsen staining was intermittently positive for Cryptosporidium, and colonic tissue histopathology revealed features of colitis, lymphocytic infiltrates, crypt abscesses, and the appearance of epithelial and cryptic basophilic spherical structures consistent with Cryptosporidium [Figure 1]. She was given meropenem for two weeks, voriconazole and caspofungin (sequentially) for four weeks, and paromomycin and clarithromycin (as antiparasitic agent nitazoxanide was not available initially) for four weeks. The bacterial and fungal infections were controlled with antimicrobial therapy. Meanwhile, she was provided total parenteral nutrition (TPN), reaching a total fluid requirement of 250 mL/kg/day. However, after four weeks of pharmacological anti-Cryptosporidium treatment and maximum nutritional support, neither diarrhea nor weight loss had improved. Her birth weight and weight at that point in time were the same (2.71 kg). Additionally, she continued to have intermittent vomiting, wet cough, tachypnea, tachycardia, and persistent high-grade fever. Therefore, disseminated cryptosporidiosis was suspected. Bronchoscopy and colonoscopy were repeated. Pulmonary and colonic cryptosporidiosis were confirmed by microscopy, cytology, and qualitative real-time PCR. Histopathology findings were consistent with cryptosporidiosis. Nitazoxanide (100 mg twice daily) was added, and paromomycin was discontinued with no apparent change in her clinical status for another eight weeks. Ultimately, she was sent to the hematopoietic stem cell transplantation (HSCT) center for primary care using a haploidentical donor. Unfortunately, she did not engraft and continued to have symptoms compatible with disseminated cryptosporidiosis with lung, hepatobiliary, and colonic involvement. She ultimately died of overwhelming sepsis at the age of eight months.
Table 1: Biochemical, microbiological, and histopathological features.
|
Hemoglobin, g/dL |
8.3 |
10.5–13.5 |
BAL culture |
Pseudomonas aeruginosa
Candida albicans |
|
Platelet count, 10*9/L |
388 |
150–450 |
Colon tissue culture |
Klebsiella pneumoniae
Enterococcus faecium |
|
White cell count, 10*9/L |
2.1 |
6–17.5 |
Blood culture |
Enterococcus faecium |
|
Lymphocytes, 10*9/L |
0.2 |
4–10.5 |
Stool viral PCR |
No virus detected. |
|
CD4+ count, cells/uL |
2.00 |
600–1200 |
Feces culture |
Negative |
|
CRP, mg/L |
73.9 |
< 5 |
Cryptosporidium stain in stool |
2/3 samples were positive |
|
ALT, IU/L |
127 |
0–40 |
Respiratory viral panel |
Positive for rhinovirus |
|
AST, IU/L |
175 |
5–35 |
HIV-1 RNA viral load (blood) |
Not detected |
|
GGT, IU/L |
148 |
10–205 |
PCR (blood, BAL) for EBV and CMV |
Not detected |
|
Albumin, g/L |
18 |
35–50 |
Adenovirus PCR (blood, BAL, and stool) |
Not detected |
|
Colonic tissue histopathology: morphological features of immunodeficiency syndrome. Colonic Cryptosporidium parvum infection. Active colitis with no granulomatous changes and no CMV or other viral inclusions. |
TB culture
(blood, BAL, tissue) |
Not detected |
|
|
CRP: c-reactive protein; ALT: alanine aminotransferase; AST: aspartate transaminase; GGT: gamma-glutamyl transferase; BAL: bronchoalveolar lavage;
EBV: Epstein-Barr virus; CMV: cytomegalovirus; TB: tuberculosis; PCR: polymerase chain reaction.
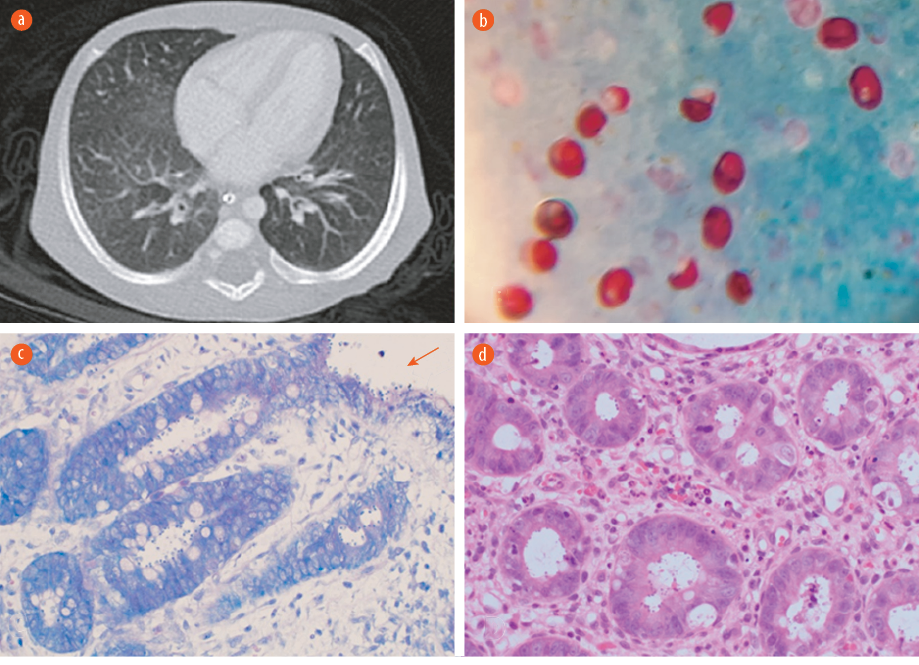
Figure 1: (a) CT scan of the chest showing scattered faint ground-glass appearance related to non-specific pulmonary infection. (b) Cryptosporidium oocyte in acid-fast stain. Oocysts appear as red, oval-shaped, in blue/green background 100 ×. (c) A modified Ziehl–Neelsen stain on low power showing the Cryptosporidium oocytes within the crypts and over the epithelial surface (red arrow) 20 ×. (d) Hematoxylin and eosin stain of colonic tissue on high power reveal features of colitis and the presence basophilic spherical organisms within the crypts, morphologically consistent with Cryptosporidium 20 ×.
Discussion
We present the first immunocompromised Omani infant with disseminated cryptosporidiosis. This Cryptosporidium infection was probably acquired by ingestion of contaminated water at a very early age. Subsequent hepatobiliary disease and aspiration of oocysts to the lungs may have occurred. However, hematogenous spread could not be ruled out. Severe T-cell deficiency contributed to the persistence and spread of the infection. Despite a high calorie/protein intake, this infant exhibited no weight gain, complicating morbidity and resulting
in mortality.
Worldwide epidemiological data on cryptosporidiosis in children with PID are limited. Aluri et al,13 recently studied 52 SCID patients, where 18 had chronic diarrhea, one of whom had cryptosporidiosis. Davies et al,14 reported that three of 42 children with PID undergoing HSCT in Northern Europe were infected with Cryptosporidium. Surveying 34 children with PID in Warsaw, Bednarska, et al,15 revealed cryptosporidiosis in two children with SCID and hyper-IgM syndrome.
The prevalence of Cryptosporidium infection in HIV-seropositive children is variable. Hunter and Nichols reviewed 18 studies, identifying a mean prevalence of 32%.11 The correlation between intestinal cryptosporidiosis and immunosuppression was studied by Legrand et al,16 where the median CD4 cell count was 60 cells/mm3 (range = 0–234) in immunocompromised individuals, and Vanathy et al,17 claimed that a CD4 count < 200 cells/mm3 is a risk factor for severe disease and dissemination.
Stool analysis was occasionally positive for Cryptosporidium in our patient. Fecal ‘ova and parasite’ -positive yield may vary because of differences in oocyst shedding, stool concentration, and the availability of experienced technical personnel.17 Meanwhile, it is crucial to involve a clinical microbiologist, employ trained staff for microscopic evaluation of at least three samples, and use molecular methods to ensure appropriate diagnostic workup and increase diagnostic sensitivity. PCR-based detection and the use of immunefluorescence microscopy are considered the gold standard for diagnosis. A nested-PCR protocol to amplify the 18S small-subunit ribosomal RNA gene is a powerful tool to identify infection in sputum, stool, and tissue.17 Mor et al,18 studied the yield of nested and restriction fragment length polymorphism PCR in respiratory secretions in children with cryptosporidiosis. A high rate of detection was demonstrated in sputum samples compared with saliva samples, highlighting the need for BAL fluid analysis for critically ill and non-expectorating children.
Histological examination and transmission electron microscopy examination has been used to diagnose Cryptosporidium infection. Tissue analysis can reveal distinct forms of the life cycle. However, missing the infected site during biopsy and the small size of the organism can lead to false-negative results.
Despite receiving antiparasitic management from diagnosis, dissemination was strongly suspected as the clinical condition failed to improve. Oral and parenteral rehydration and correction of the acid-base imbalance and electrolyte disturbances are the mainstay of supportive care in intestinal cryptosporidiosis, with TPN and antidiarrheal compounds as additional supportive measures usually providing a good outcome. Antiparasitic treatment might be of modest effectiveness in immunocompetent children. However, the efficacy of such agents in children with immunodeficiency may be limited. There is no recommended dose for antiparasitic agents in children with PID. Nitazoxanide, an antiprotozoal, and first-in-class broad-spectrum antiviral, is an Food and Drug Administration approved treatment for cryptosporidiosis in immunocompetent children. However, its effectiveness and dosing regimen remain poorly delineated in this group of patients. Alternative treatment options include paromomycin, clofazimine, and add-on therapy with azithromycin or clarithromycin.19 For our patient, antiparasitic treatment with paromomycin and nitazoxanide with the addition of a macrolide did not control the infection or improve intestinal and pulmonary symptoms despite treatment for about three months. A meta-analysis found no reduction in: 1) the duration of diarrhea, 2) mortality, and 3) parasitological clearance when using either nitazoxanide or paromomycin in HIV-seropositive patients.20 Legrand et al,16 observed resolution of diarrhea after a mean five-week course of nitazoxanide and azithromycin in three children with intestinal cryptosporidiosis receiving HSCT for PID. However, their median CD4 number increased to 513 (133–615) at the end of therapy, indicating the importance of immune function restoration in addition to antimicrobial therapy.Antiviral therapy in HIV-seropositive children with intestinal cryptosporidiosis can result in immune restoration and parasite eradication similar to children with PID undergoing HSCT.21 Recently, a piperazine-based lead compound showed effective Cryptosporidium elimination in highly immunocompromised non-obese diabetic scid gamma mice.12 This holds promise for effective pharmacological treatment while awaiting immune reconstitution in children with primary and secondary immunodeficiency status.
Conclusion
In Oman, children with suspected or confirmed PID should consume sterile water to decrease the risk of protozoal infection. Cryptosporidiosis in the context of immunodeficiency is of great concern. Therefore, high awareness and urgent action are critical, especially when the clinical condition is deteriorating. Utilization of PCR techniques and consulting expertise in microbiological diagnostics in centers managing children with immunodeficiency are crucial. Urgent reconstitution of immune function in children with PID appears conducive to a positive outcome for disseminated cryptosporidiosis compared with pharmacological therapy alone.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from the parents of the patient.
references
- 1. Al Awaidy ST, Sharanya A. Successes and challenges of HIV/AIDS program in Oman: 1984-2015. Oman Med J 2019 Jan;34(1):1-8.
- 2. Al-Tamemi S, Naseem SU, Al-Siyabi N, El-Nour I, Al-Rawas A, Dennison D. Primary immunodeficiency diseases in Oman: 10-year experience in a tertiary care hospital. J Clin Immunol 2016 Nov;36(8):785-792.
- 3. Windsor JJ, El-Shafie S, Welch S, Al-Toqui F. Cryptosporidiosis in children from the Sultanate of Oman. Sultan Qaboos Univ Med J 1999;1(1):59-62.
- 4. Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s principals and practices of infectious diseases. Elsevier Saunders; Philadelphia, PA. Cryptosporidiosis (Cryptosporidium Species); 2015 p. 3173–3183.
- 5. Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 2015 Jan;15(1):85-94.
- 6. Cama VA, Bern C, Roberts J, Cabrera L, Sterling CR, Ortega Y, et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis 2008 Oct;14(10):1567-1574.
- 7. Castellanos-Gonzalez A, Martinez-Traverso G, Fishbeck K, Nava S, White AC Jr. Systematic gene silencing identified Cryptosporidium nucleoside diphosphate kinase and other molecules as targets for suppression of parasite proliferation in human intestinal cells. Sci Rep 2019 Aug;9(1):12153.
- 8. Current WL, Garcia LS. Cryptosporidiosis. Clin Microbiol Rev 1991 Jul;4(3):325-358.
- 9. Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology 2014 Nov;141(13):1667-1685.
- 10. Chalmers RM, Katzer F. Looking for Cryptosporidium: the application of advances in detection and diagnosis. Trends Parasitol 2013 May;29(5):237-251.
- 11. Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev 2002 Jan;15(1):145-154.
- 12. Jumani RS, Bessoff K, Love MS, Miller P, Stebbins EE, Teixeira JE, et al. A novel piperazine-based drug lead for cryptosporidiosis from the medicines for malaria venture open-access malaria box. Antimicrob Agents Chemother 2018; 27;62(4): e01505-17.
- 13. Aluri J, Desai M, Gupta M, Dalvi A, Terance A, Rosenzweig SD, et al. Clinical, immunological, and molecular findings in 57 patients with severe combined immunodeficiency (SCID) from India. Front Immunol 2019 Feb;10:23.
- 14. Davies AP, Slatter M, Gennery AR, Robinson G, Crouch N, Elwin K, et al. Prevalence of cryptosporidium carriage and disease in children with primary immune deficiencies undergoing hematopoietic stem cell transplant in Northern Europe. Pediatr Infect Dis J 2017 May;36(5):504-506.
- 15. Bednarska M, Jankowska I, Pawelas A, Piwczyńska K, Bajer A, Wolska-Kuśnierz B, et al. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res 2018 Sep;117(9):2869-2879.
- 16. Legrand F, Grenouillet F, Larosa F, Dalle F, Saas P, Millon L, et al. Diagnosis and treatment of digestive cryptosporidiosis in allogeneic haematopoietic stem cell transplant recipients: a prospective single centre study. Bone Marrow Transplant 2011 Jun;46(6):858-862.
- 17. Vanathy K, Parija SC, Mandal J, Hamide A, Krishnamurthy S. Cryptosporidiosis: a mini review. Trop Parasitol 2017 Jul-Dec;7(2):72-80.
- 18. Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, et al. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clinic Infect Dis. 2010;15;50(10):1366-1372.
- 19. Ritchie DJ, Becker ES. Update on the management of intestinal cryptosporidiosis in AIDS. Ann Pharmacother 1994 Jun;28(6):767-778.
- 20. Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol 2007 Apr;63(4):387-393.
- 21. Barboni G, Candi M, Inés Villacé M, Leonardelli A, Balbaryski J, Gaddi E. Intestinal cryptosporidiosis in HIV infected children. Medicina (B Aires) 2008;68(3):213-218.