Humanity has borne an unprecedented health burden due to the COVID-19 pandemic, ever since the first case was reported on December 2019 in Wuhan, China.1 Oman itself has recorded approximately 389 000 cases and 4285 deaths till April 2022.2
COVID-19 is caused by a novel SARS-CoV-2.1 Seven members of the coronavirus family are known to cause human illnesses. Of these, four (i.e., 229E, NL63, OC43, and HKU1) have been long associated with various forms of common cold. Three later-identified strains (MERS-CoV, SARS-CoV, and SARS-CoV-2) are attributed to severe human diseases.3 SARS-CoV-2, the last to be identified, is a single-stranded RNA virus characterized by its high transmission rates compared to the other coronavirus family members.4,5
Depending on the clinical presentation, the diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) classifies COVID-19 cases into four categories: mild, moderate, severe, and critical.6 The severity and progression of COVID-19 are associated with changes in multiple biomarkers including hematological, biochemical, and immunological markers.7–10 Early in the pandemic, a meta-analysis of 21 studies comprising a total of 3377 patients and 33 laboratory parameters concluded that the progression of COVID-19 cases to the critical presentation was predicted by platelets count, white cell count (WCC), lymphocyte count, ferritin, and interleukin-6 (IL-6) levels.9 This was followed by more studies that investigated other possible predictors for the disease progression and severity, including C-reactive protein (CRP), procalcitonin, cytokines, coagulation parameters, lactate dehydrogenase (LDH), liver enzymes, and cardiac biomarkers.11–15 The current study aimed to identify possible predictors of intensive care admission for COVID-19 in a cohort of patients from Oman.
MethodS
Clinical data (on admission) of all PCR-confirmed COVID-19 cases admitted to the Royal Hospital (a tertiary hospital), Muscat, Oman from 24 February to 31 July 2020 were retrospectively retrieved. This included demographic information, clinical history details (i.e., signs, symptoms, contact history, radiographic findings, hospitalization duration, and comorbidities), and laboratory results including complete blood count, coagulation parameters, liver profile, LDH, cardiac markers, and inflammatory markers. Progression severity of COVID-19 was assessed by admission to intensive care units (ICUs) based on clinical management of the patient. The ICU admission was based on oxygen peripheral saturation (SpO2) and/or dyspnea and/or mental confusion. This study was approved by the Research and Ethical Review and Approve Committee (RERAC) at Royal Hospital, Ministry of Health (SRC#110/2020).
To identify the predictors of severity of COVID-19, we categorized the patients into ICU and non-ICU groups. The inclusion criteria included adults and children with a confirmed diagnosis of COVID-19 by molecular tests and patients with available baseline clinical and laboratory data. Patients who had primary or secondary immune deficiency were excluded from the study.
The data were analyzed using SPSS windows (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). In the case of categorized variables, the chi-square test was used. For continuous variables that followed the normal distribution pattern, the analysis of variance was used to compare means. Otherwise, Mann–Whitney U-test was used in the variables that did not follow the normal distribution. A p-value of < 0.050 was considered significant. The predictability of studied variables was determined using receiver operating characteristic (ROC) curves followed by Youden index to identify the best predicting cut-off value for each biomarker.
Results
Out of 445 patients, 276 (62.0%) were male with a median age of 49 (18–87) years and 169 (38.0%) were female with a median age of 53 (15–94) years. Omani patients represented 55.7% (248 patients) of the study subjects. Of the patients in the study population, 46.1% had diabetes mellitus (DM), 46.7% had hypertension (HTN), and 24.0% had dyslipidemia. In addition, 11.0% of patients reported respiratory problems, 18.4% cardiac problems, 8.3% liver disease, and 20.2% chronic kidney disease (CKD). Table 1 summarizes the demographics and other characteristics of the COVID-19 patients based on ICU admission.
Table 1: Demographics and characteristics of patients with COVID-19 admitted in non-ICU and ICU wards.
|
Demographics
|
|
|
|
|
|
No. of patients
|
445
|
259 (58.2)
|
186 (41.8)
|
-
|
|
Male
|
276 (62.0)
|
152 (55.1)
|
124 (44.9)
|
-
|
|
Female
|
169 (38.0)
|
107 (63.3)
|
62 (36.7)
|
-
|
|
Omani nationals
|
248 (55.7)
|
186 (75.0)
|
62 (25.0)
|
< 0.001
|
|
Comorbidities
|
|
|
|
|
|
Diabetes
|
205 (46.1)
|
103 (50.2)
|
102 (49.8)
|
0.002
|
|
Hypertension
|
208 (46.7)
|
123 (59.1)
|
85 (40.9)
|
NS
|
|
Dyslipidemias
|
107 (24.0)
|
59 (55.1)
|
48 (44.9)
|
NS
|
|
Respiratory disease
|
49 (11.0)
|
21 (42.9)
|
28 (57.1)
|
0.031
|
|
Cardiac conditions
|
82 (18.4)
|
45 (54.9)
|
37 (45.1)
|
NS
|
|
Liver disease
|
37 (8.3)
|
15 (40.5)
|
22 (59.5)
|
0.035
|
ICU: intensive care unit; NS: not significant.
Patients admitted to ICU had a higher mortality (OR = 3.3; 95% CI: 2.0–5.6), sepsis (OR = 3.5; 95% CI: 1.6–7.4), intubation (OR = 31.2; 95% CI: 18.2–53.7), and acute respiratory distress syndrome (OR = 41.5; 95% CI: 20.4– 84.8) [Figure 1].
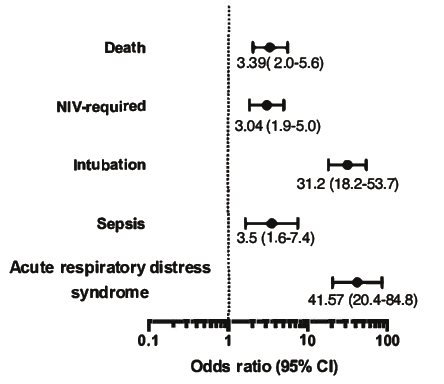 Figure 1: Complications associated with intensive care unit admission with odds ratio (95% CI)
Figure 1: Complications associated with intensive care unit admission with odds ratio (95% CI)
for each.
Out of 445 patients, 259 (58.2%) were admitted into COVID-19 wards and 186 (41.8%) into ICU. Figure 2 summarizes the association of comorbidities at the time of diagnosis upon admission to ICU. Patients with liver diseases presented an OR = 2.1; 95% CI: 1.1–4.3. Furthermore, the following patients were significantly more likely to be admitted to ICU: DM (OR = 1.8; 95% CI: 1.3–2.7) and respiratory diseases (OR = 2.0; 95% CI: 1.1–3.7). In contrast, having HTN, CKD, cardiac conditions, and dyslipidemia as comorbidities at admission were not associated with higher risk to be admitted to ICU.
 Figure 2: On-admission comorbidities associated with intensive care unit admission with odds ratio (95% CI) for each comorbidity.
Figure 2: On-admission comorbidities associated with intensive care unit admission with odds ratio (95% CI) for each comorbidity.
We then studied other parameters in patients with COVID-19 associated with ICU admission [Figure 3]. The age of patients was not associated with ICU admission (51.0±16.0 years vs. 50.0±14.0). Longer hospital stay was significantly associated with ICU admission (8.3±9.1 days vs. 17.5±12.9; p < 0.001) [Figure 3]. In addition, on admission high values of each of the following parameters: WCC, CRP, ferritin, IL-6, D-dimer, alanine aminotransferase (ALT), LDH, and troponin were significantly associated with ICU admission (p < 0.001 for all parameters except troponin; p < 0.050). Furthermore, low values for lymphocyte count, corrected calcium levels, and albumin were markedly associated with ICU admission (p < 0.001 for all three parameters), Figure 3. Levels of vitamin D in patients were not associated with ICU admission.
 Figure 3: Factors associated with intensive care unit admission, age, hospitalization period, and biomarkers.
Figure 3: Factors associated with intensive care unit admission, age, hospitalization period, and biomarkers.
We used the receiver operating characteristic curve to assess the predictivity of each significant biomarker in Figure 3. Figures 4 and 5 show the area under the curve (AUC), optimum cut-off point, and OR of admission biomarkers. LDH levels of > 419.5 IU/L was the most predictive biomarker for ICU admission with an AUC of 78% (OR = 7.3; 95% CI: 4.6–11.5).
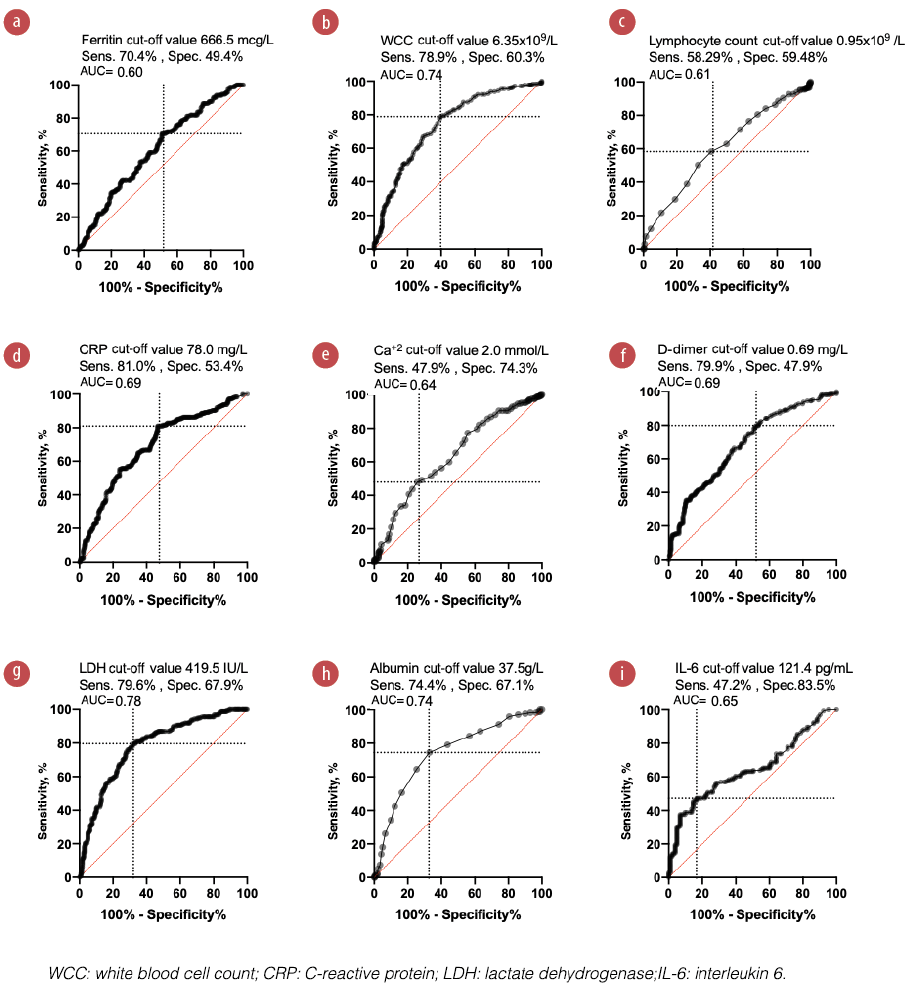 Figure 4: Receiver operator characteristic curves presenting the predictive potential of different admission biomarkers to predict COVID-19 progression.
Figure 4: Receiver operator characteristic curves presenting the predictive potential of different admission biomarkers to predict COVID-19 progression.
 Figure 5: Forest plot showing odds ratio (95% CI) of on-admission biomarkers associated with intensive care unit admission.
Figure 5: Forest plot showing odds ratio (95% CI) of on-admission biomarkers associated with intensive care unit admission.
In addition, the other biomarkers showed variable significant predictivity as follows: WCC > 6.35 × 109/L, AUC = 74% (OR = 5.3, 95% CI: 3.5–8.2), albumin < 37.5 g/L, AUC = 74% (OR = 5.3, 95% CI: 3.5–8.0); IL-6 > 121.4 pg/mL, AUC = 65% (OR = 4.53, 95% CI: 2.4–8.5); CRP > 78.0 mg/L, AUC = 69% (OR = 4.5, 95% CI: 2.9–6.9); D-dimer > 0.69 mg/L, AUC = 69% (OR = 3.4, 95% CI: 2.1–5.4); ferritin > 666.5 mcg/L, AUC = 60% (OR = 2.3, 95% CI: 1.5–3.6); corrected Ca < 2.0 mmol/L, AUC = 64% (OR = 2.2, 95% CI:1.4–3.4; and lymphocytes < 0.95 × 109/L, AUC 61% (OR = 1.9, 95% CI: 1.3-2.8).
AUC, sensitivity, specificity, and optimum critical cut-off values are shown. AUC ≥ 0.6 was considered significant. Youden’s index was used to find out optimum cut-off values.
Discussion
The ability to predict the progression and severity of COVID-19 in a cohort of patients greatly facilitates patient management. Therefore, many studies sought to identify the predictive factors that could be applied in clinical practice. Comorbidities such as DM, HTN, liver disorders, respiratory diseases, renal illnesses, immunodeficiencies, and malignancies were studied extensively.16–18 Very early in the pandemic data (mid-2020) from China suggested that the likelihood for severe COVID-19 presentation was higher in individuals with HTN, DM, respiratory diseases, and cardiovascular diseases with OR > 2.30.19 A very recent study (mid-2022), from India found that > 98% of patients who died from COVID-19 had HTN or DM.20 Other studies delved deeper to find out more predictive biomarkers for COVID-19 progression and investigated LDH, WCC, CRP, D-dimer, ferritin, lymphocytes, procalcitonin, electrolytes, cardiac enzymes, hepatic enzymes, cytokines (IL-6, interleukin 10 (IL-10), and etc.), coagulation, and others.12,21–23 Indeed, it has been found that mortality from COVID-19 is likely to be associated with leukocytosis, neutrophilia, lymphocytopenia, high urea, and creatinine, as well as high levels of CRP, LDH, ferritin, and D-dimer.22
In the current study, we found that 41.8% of COVID-19 patients were admitted to ICU, which is much higher than the findings of Guan et al,24 where only 5% of 1099 patients were admitted to ICU. However, this can be attributed to the difference in the sample sizes and the fact that our study used data from a single center. Moreover, we identified that certain preexisting comorbidities including DM, liver diseases, and respiratory diseases were significantly associated with COVID-19 progression that required ICU admission. On the other hand, HTN, CKD, cardiac conditions, and dyslipidemia were not predictors of ICU admission. A previous meta-analysis showed that DM, malignancy, cerebrovascular disease, respiratory system disease, hepatitis B infection, and digestive diseases were markedly associated with COVID-19 severity.17 Unlike our present results, Fang,17 found that age, HTN, cardiac diseases, and CKD were significant contributors to disease severity that can be related to the sample size, demographics, and multiple coexisting comorbidities. Cardiac disorders (i.e. myocarditis, myocardial infarction, and exacerbated heart failure) are also reported to be associated with severe COVID-19.25
The effect of DM on COVID-19 severity can be attributed to multiple factors including the presence of DM complications and increased expression of both angiotensin-converting enzyme 2 and dipeptidyl peptidase 4.16,26,27 Besides altered immune function, dipeptidyl peptidase 4 has been noticed in increased concentrations in some hepatic disorders which as well can explain the relationship between liver illnesses and COVID-19 progression.16,28 Apparently, raised expression of some molecules (i.e., SLC2A1 (GLUT1), SLC7A5(CD98), transmembrane protease serine 2, ITGA3, and ITGA6), a limited pulmonary reserve and restrictive ventilatory impairment associated with pulmonary disorders can elucidate the progression of COVID-19 patient to ICU admission.28
Our study showed that multiple biomarkers have noticeable predictivity as indicators for COVID-19 progression to critical levels that need ICU admission. Current results revealed LDH > 419.5 IU/L as the most predictive biomarker for ICU admission with OR = 7.3 (95% CI: 4.6–11.5). Our finding is consistent with previous studies that reported LDH as the strongest predictor for COVID-19 severity.22,23
Likewise, WCC > 6.35 × 109/L, albumin < 37.5 g/L, IL-6 > 121.4 pg/mL, CRP > 78.0 mg/L, D-dimer > 0.69 mg/L, ferritin > 666.5 mcg/L, corrected Ca < 2.0 mmol/L, and lymphocytes < 0.95 × 109/L were all significantly associated with COVID-19 progression and ICU admission. Since the beginning of the pandemic, many studies showed that the above-mentioned biomarkers were markedly associated with COVID-19 progression, severity, and fatality. Henry et al,9 demonstrated the significant association of high WCC, IL-6, IL-10, ferritin, cardiac enzymes, coagulation indicators, decreased lymphocyte, and platelet counts to both severe and fatal COVID-19. Wang L et al,29 showed a noticeable correlation between increased CRP levels and COVID-19 severity. Several other studies revealed remarkable associations of the following biomarkers with COVID-19 progression: Tan et al,30 (CRP and erythrocyte sedimentation rate (ESR)), Zeng et al,12 (CRP, procalcitonin (PCT), IL-6, and ESR), Zhang et al,31 (D-dimer), Gao et al,28 (IL-6 and D-dimer), Pan et al,21 (lymphocyte, CRP, PCT, and LDH), Asghar et al,22 (LDH, PCT, D-dimer, CRP, and ferritin), Han et al,32 (IL-6 and IL-10), Lagadinou et al,33 (LDH, D-dimers, CRP, fibrinogen, and ferritin), and Liu et al,34 (IL-6 and CRP).
Although the markers associated with COVID-19 severity have been reported widely in the literature, our study has uniquely identified the cut-off values for these biomarkers based on data from Oman. The limitation of the study is being a retrospective cohort study, data were collected from those already admitted to the hospital and may not include all COVID-19 variants. Another limitation is that our study is based on data from a single tertiary center, while Oman has several tertiary centers that catered to advanced cases of COVID-19.
Conclusion
This study identified that DM, liver diseases, and respiratory diseases, as comorbidities are associated with higher admission to ICU in patients with COVID-19. Furthermore, our study has uniquely identified the cut-off values for some on-admission laboratory blood and serum parameters (i.e., WBC, lymphocytes, CRP, ferritin, corrected calcium, IL-6, D-dimer, ALT, LDH, albumin, and troponin) for predicting ICU admission in patients with COVID-19.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Sharma S, Shrivastava S, Kausley SB, Rai B, Pandit AB. Coronavirus: a comparative analysis of detection technologies in the wake of emerging variants. Infection 2022 Apr;2022:1-19.
- 2. Khamis F, Al Rashidi B, Al-Zakwani I, Al Wahaibi AH, Al Awaidy ST. Epidemiology of COVID-19 infection in Oman: analysis of the first 1304 cases. Oman Med J 2020 Jun;35(3):e145-e145.
- 3. Wojtysiak K, Zielińska-Więczkowska H. Work in stressful conditions in medical emergency system during the COVID-19 pandemic. Med Pr 2022 Apr;147840.
- 4. Ludwig S, Zarbock A. Coronaviruses and SARS-CoV-2: a brief overview. Anesth Analg 2020 Jul;131(1):93-96.
- 5. Balkhair AA. COVID-19 pandemic: a new chapter in the history of infectious diseases. Oman Med J 2020 Apr;35(2):e123-e123.
- 6. Wei P-F. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl) 2020 May;133(9):1087-1095.
- 7. Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 2020 Jun;95:304-307.
- 8. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Feb;395(10223):497-506.
- 9. Henry BM, de Oliveira MH, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020 Jun;58(7):1021-1028.
- 10. Li ZB, Liu J, Zhang SQ, Yu Y, Liang HF, Lu QQ, et al. Novel potential metabolic biomarker panel for early detection of severe COVID-19 using full-spectrum metabolome and whole-transcriptome analyses. Signal Transduct Target Ther 2022 Apr;7(1):129.
- 11. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci 2020 Aug;254:117788.
- 12. Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis 2020 Jul;96:467-474.
- 13. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci 2020 Sep;57(6):389-399.
- 14. Assandri R, Accordino S, Canetta C, Buscarini E, Scartabellati A, Tolassi C, et al. Long pentraxin 3 as a marker of COVID-19 severity: evidences and perspectives. Biochem Med (Zagreb) 2022 Jun;32(2):020901.
- 15. Díaz-Troyano N, Gabriel-Medina P, Weber S, Klammer M, Barquín-DelPino R, Castillo-Ribelles L, et al. Soluble angiotensin-converting enzyme 2 as a prognostic biomarker for disease progression in patients infected with SARS-CoV-2. Diagnostics (Basel) 2022 Apr;12(4):886.
- 16. Bassendine MF, Bridge SH, McCaughan GW, Gorrell MD. COVID-19 and comorbidities: a role for dipeptidyl peptidase 4 (DPP4) in disease severity? J Diabetes 2020 Sep;12(9):649-658.
- 17. Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020 Jul;12(13):12493-12503.
- 18. Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 2021 Feb;76(2):428-455.
- 19. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020 May;94:91-95.
- 20. SaifAndrabi SM. Clinical profile and outcome of COVID-19 patients along with the impact of comorbidities on the disease outcome at the secondary care hospital of Union Territory of J&K. J Assoc Physicians India 2022 Apr;70(4):11-12.
- 21. Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci 2020 May;17(9):1281-1292.
- 22. Asghar MS, Haider Kazmi SJ, Khan NA, Akram M, Hassan M, Rasheed U, et al. Poor prognostic biochemical markers predicting fatalities caused by COVID-19: a retrospective observational study from a developing country. Cureus 2020 Aug;12(8):e9575.
- 23. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci 2020 Mar;24(6):3404-3410.
- 24. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020 Apr;382(18):1708-1720.
- 25. Gaze DC; Annals of Clinical Biochemistry. Clinical utility of cardiac troponin measurement in COVID-19 infection. Ann Clin Biochem 2020 May;57(3):202-205.
- 26. Zemlin AE, Wiese OJ; Annals of Clinical Biochemistry. Coronavirus disease 2019 (COVID-19) and the renin-angiotensin system: a closer look at angiotensin-converting enzyme 2 (ACE2). Ann Clin Biochem 2020 Sep;57(5):339-350.
- 27. Filardi T, Morano S. COVID-19: is there a link between the course of infection and pharmacological agents in diabetes? J Endocrinol Invest 2020 Aug;43(8):1053-1060.
- 28. Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020 Jul;92(7):791-796.
- 29. Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 2020 Jun;50(4):332-334.
- 30. Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol 2020 Jul;92(7):856-862.
- 31. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020 Jun;18(6):1324-1329.
- 32. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 2020 Dec;9(1):1123-1130.
- 33. Lagadinou M, Salomou EE, Zareifopoulos N, Marangos M, Gogos C, Velissaris D. Prognosis of COVID-19: changes in laboratory parameters. Infez Med 2020 Jun;28(suppl 1):89-95.
- 34. Liu R, Ma Q, Han H, Su H, Liu F, Wu K, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med 2020 Jun;58(7):1121-1124. CCLM.