Dengue is a viral infection transmitted to humans from the bite of infected mosquitoes. The principal vector is the Aedes aegypti mosquito and, to a lesser degree, Aedes albopictus which are found in the tropical and subtropical regions across the world, as well as in some temperate parts of Africa, Europe, the Middle East, and North and South America.1
Dengue fever (DF) is mostly a self-limiting disease with clinical features reminiscent of a common viral disease. However, some patients may develop dengue hemorrhagic fever (DHF), which is a serious and potentially fatal illness.2
Arboviruses that are transmitted by arthropods causing diseases such as DF, yellow fever, chikungunya, and Zika are considered major international public health risks and serious health threats in the tropical and subtropical areas where approximately 3.9 billion people reside. The incidence and magnitude of arboviruses outbreaks, particularly those transmitted by Aedes aegypti mosquitoes, are increasing, fueled by the convergence of ecological, economic, and social factors.3
Over the last several decades, dengue viruses have moved fast across regions resulting in an increase in the frequency of outbreaks and the emergence of serotypes that are associated with severe DF. The fight against arboviruses is at the top of the World Health Organization priority list with growing evidence that their risk is on the rise.4 Every year, at least 60 million symptomatic infections occur worldwide, with roughly 10 000 fatalities. Approximately more than 4 billion people are in danger in countries where Aedes spp. mosquitoes thrive.4 Moreover, more than 129 countries have reported DF cases,5 with 70% of the true burden being in Asia.6
In Oman, between 2001 and 2017, 95% of DF cases were among adults and were travel-associated. Overall case counts and rates have increased between 2013 and 2020 [Figure 1] and most cases of DF were reported in persons over 25 years of age [Figure 2].
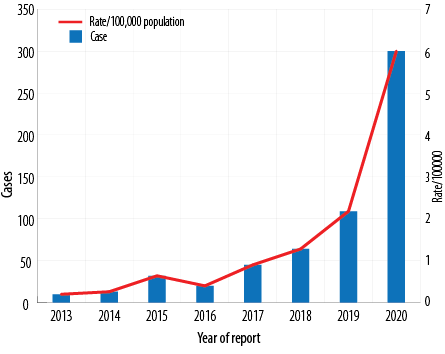 Figure 1: Dengue fever reported and rate per 100 000 population in Oman from 2013 to 2020.
Figure 1: Dengue fever reported and rate per 100 000 population in Oman from 2013 to 2020.
 Figure 2: Dengue cases by age in Oman from 2013 to2020.
Figure 2: Dengue cases by age in Oman from 2013 to2020.
Although, Aedes aegypti and Aedes albopictus have been found in Oman since 2008, autochthonous (indigenous) dengue transmission was not previously described. However, an outbreak of DF was reported from December 2018 to January 2019 with no travel history of the index case, confirming the occurrence of autochthonous and the first-ever indigenous dengue transmission. The reported incidence rate per 100 000 population of total suspected cases was 2.34, and confirmed cases locally acquired was 0.41.7 Entomological surveys yielded the vector Aedes aegypti in A´Seeb, Bawshar, and Muttrah in Muscat governorate where most of DF cases were reported.8 The outbreak was effectively contained due to rapid response and extensive community-wide vector control activities. Notwithstanding surveillance systems; DF and DHF are reportable diseases and vector control programs are in place in the country.
On 22 May 2022, the health authorities detected the first re-emergent case of DF. Since then, a total of 127 cases have been reported. Of these, 114 (90%) were in Muscat governorate. All patients had dengue virus serotype 2. The purpose of this study is to describe the epidemiological and clinical characteristics of patients presenting with DF at the Royal Hospital, the main tertiary care hospital in Oman.
Methods
We conducted a retrospective cohort study between 1 January and 18 April 2022 and included all patients with laboratory-confirmed DF. Patients were tested for DF based on the national case definitions of suspected and confirmed DF.9
The data were obtained from the hospital medical records that included the baseline demographic characteristics (gender, age, occupation, place of residency, and nationality), information on the date of onset of illness, date of DF confirmation, travel history, contact history, symptoms on presentation, risk factors and underlying comorbidities, physical signs on presentation, laboratory parameters, and patients’ outcomes.
DF was defined as an acute onset of fever of less than three weeks duration in a severely ill patient and any two of the following: hemorrhagic or purpuric rash, epistaxis, hematemesis, hemoptysis, blood in stool, other hemorrhagic symptoms, and no known predisposing host factors for hemorrhagic manifestations.9 Thrombocytopenia was classified as mild if the platelet count is between 100 000–150 000 per microliter (mcL), moderate if the platelet count is between 50 000–100 000 per mcL, and severe if the platelet count is < 50 000 per mcL.
For non-admitted patients and symptomatic patients for more than seven days, blood samples were tested by enzyme-linked immunosorbent assay (Bioline™ Dengue Duo, Ingbert, Germany) to detect the nonstructural protein 1, immunoglobulin M, and immunoglobulin G. Admitted patients with symptoms less than seven days were tested by reverse transcription polymerase chain reaction assay and/or serology.
The data was described using descriptive statistics. For categorical variables, frequencies and percentages were reported. The mean and SD were used to summarize the data for continuous variables. Statistical analyses were performed using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.).
The study was approved by the Royal Hospital Scientific Research Committee (SRC#47/2022). The data collected did not include human subjects in terms of interviews. The researchers used extant data and information extracted and published in an official domain and in adherence to the Declaration of Helsinki.
Results
The study included 58 patients with laboratory- confirmed DF who were seen in the hospital. Of those, 67.2% (n = 39) were admitted to the hospital. The overall mean age of the cohort was 41.0±20.0 years ranging from 5–81 years and 55.2% (n = 32) were females [Table 1]. Most of the cases were Omani (86.2%; n = 50) with the second largest nationality being Indians (10.3%; n = 6). Eighty-one percent (n = 47) of patients were residents of Bawshar, a wilayat in Muscat governorate. All had no recent travel history except two patients, one had returned from Tanzania while the other one returned from Iran.
Table 1: Demographic and clinical characteristics on admission.
|
Age
|
58
|
|
|
Mean ± SD, years
|
|
41.0 ± 20.0
|
|
Absolute range, years
|
|
5–81
|
|
Gender
|
58
|
|
|
Male
|
|
26 (44.8)
|
|
Female
|
|
32 (55.2)
|
|
Nationality
|
58
|
|
|
Oman
|
|
50 (86.2)
|
|
Indian
|
|
6 (10.3)
|
|
Taiwan
|
|
1 (1.7)
|
|
Bangladesh
|
|
1 (1.7)
|
|
Wilayat (district)
|
58
|
|
|
Bawshar
|
|
47 (81.0)
|
|
A'Seeb
|
|
10 (17.2)
|
|
Nizwa
|
|
1 (1.7)
|
|
Comorbidities
|
58
|
|
|
Hypertension
|
|
17 (29.3)
|
|
Diabetes mellitus
|
|
10 (17.2)
|
|
Ischemic heart disease
|
|
4 (6.9)
|
|
Admitted
|
58
|
39 (67.2)
|
|
On admission, mean ± SD
|
|
|
|
Systolic BP, mean ± SD, mmHg
|
58
|
120.0 ± 22.0
|
|
Diastolic BP, mean ± SD, mmHg
|
58
|
71.0 ± 15.0
|
|
Heart rate, mean ± SD, bpm
|
58
|
88.0 ± 16.0
|
|
Hemoglobin, mean ± SD, g
|
58
|
13.0 ± 1.9
|
|
WBC, mean ± SD, × 109/L
|
58
|
3.2 ± 1.7
|
|
Platelets, mean ± SD, × 109/L
|
58
|
110.0 ± 75.0
|
|
Total bilirubin, mean ± SD, μmol/L
|
51
|
11.5 ± 6.7
|
|
Bicarbonate, mean ± SD, mmol/L
|
47
|
25.7 ± 3.3
|
|
Urea, mean ± SD, mmol/L
|
56
|
5.0 ± 3.4
|
|
Serum creatinine, mean ± SD, μmol/L
|
57
|
81.0 ± 29.0
|
BP: blood pressure; WBC: white blood cell; bpm: beats per minute ; eGFR: estimated glomerular filtration rate.
None of the patients had a past history of DF. The two most prominent comorbidities were hypertension (29.3%; n = 17) and diabetes mellitus (17.2%; n = 10). On admission, most of the mean blood pressure measurements, and hematological and biochemical parameters were within the normal limits. The two most prevalent complications were increased risk of bleeding (platelet count < 50 000/mm3; 31.0%; n = 18) and hepatic impairment (15.5%; n = 9) [Table 2]. Out of the total cases, 27.6% (n = 16) of the patients were on antibiotics with most of them on ceftriaxone as an antimicrobial of choice. The three most prevalent presenting symptoms on presentation were fever (98.3%; n = 57), muscle aches and pains (55.2%; n = 32), and headache (53.4%; n = 31) and none developed DHF. The other dengue symptoms of the cohort are outlined in Figure 3. In the present outbreak, dengue virus 2 was the predominant serotype. There were no fatalities among the patients during the study period.
Table 2: Complications and management of the cohort.
|
Complications
|
|
|
|
Neuro-dysfunction
|
58
|
1 (1.7)
|
|
Hepatic impairment
|
58
|
9 (15.5)
|
|
Platelet count < 50 000/mm3
|
58
|
18 (31.0)
|
|
Pleural effusion
|
58
|
1 (1.7)
|
|
Gastrointestinal hemorrhage
|
58
|
1 (1.7)
|
|
Management
|
|
|
|
Intravenous fluids
|
57
|
44 (77.2)
|
|
Antibiotics
|
58
|
16 (27.6)
|
|
Ceftriaxone
|
|
7 (12.1)
|
|
Amoxicillin/clavulanic acid
|
|
2 (3.4)
|
|
Ceftriaxone + azithromycin
|
|
1 (1.7)
|
|
Ceftriaxone + vancomycin
|
|
2 (3.4)
|
|
Ceftriaxone + metronidazole
|
|
1 (1.7)
|
|
Ciprofloxacin
|
|
1 (1.7)
|
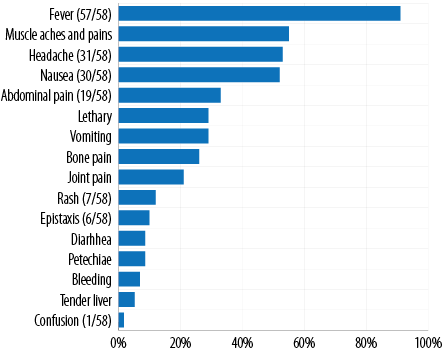 Figure 3: Presenting signs and symptoms of dengue cases admitted to the Royal Hospital, N = 58.
Figure 3: Presenting signs and symptoms of dengue cases admitted to the Royal Hospital, N = 58.
Discussion
Aedes aegypti and Aedes albopictus presence has been documented in several locations of Oman since 2008, yet autochthonous dengue transmission has not been extensively reported until 2018. Since several autochthonous outbreaks have been recorded.8 The original introduction of the dengue virus might be attributed to non-Omani-citizens returning from epidemic/endemic tropical and subtropical countries favoring the importation of the virus into the country. Similar importations of the dengue virus were observed in Italy that posed a risk for subsequent dengue outbreaks in Italy10 as well as other European countries.11–13 This recent local outbreak of DF in Oman has confirmed autochthonous transmission as all the cases had no history of traveling except two. Globalization and global warming have facilitated its expansion to nonendemic countries over the years.14
Aedes aegypti remains the primary vector for dengue transmission in Oman, similar to what was identified in western Saudi Arabia.15
Dengue transmission is influenced by environmental conditions, as seen by the disease’s seasonality.16,17 Factors that can impact mosquito reproduction and feeding habits and dengue virus incubation times can contribute to the rise in cases such as high mosquito population levels, sensitivity to circulating DFV serotypes, favorable air temperatures (25–30 °C), precipitation, and humidity.18
Likewise, DF outbreaks in Oman follow a seasonal pattern, with transmission increasing in the winter months. Based on previous observations of autochthonous arbovirus transmission in the past years, sporadic autochthonous cases or limited clusters of dengue cases are expected in the country, primarily in the winter and autumn season in Dhofar governorate (southern province) due to established populations of a competent vector (Aedes aegypti) and the tropical climate where temperatures range between 20 °C–30° C.
However, lately, from November to February, tropical environmental conditions are seen in Muscat (northern province) with the mean temperatures fluctuating between 24–28°C as well as high humidity. This provides the ideal setting for Aedes aegypti and other mosquitos breeding, therefore sustaining dengue transmission.18 During these favorable climatic circumstances, the mosquito circulates often in locations with inadequate mosquito control, and imported dengue viruses may infect the abundant vector populations. A study from Singapore indicated that mean temperatures under around 28 °C are associated with an increase in dengue incidence in the urbanized tropical city.19
Other elements that might aid indigenous transmission include some local habits and practices, such as the use of water containers that enhance mosquito breeding and increase mosquito density. Furthermore, there has been an extraordinary growth in the number of travelers from endemic areas flying to the country in the last several years, as well as the importation of commodities from dengue-endemic countries such as tires, containers, and plants. All of these factors may contribute to the transmission and spread of the invasive mosquitos Aedes aegypti and Aedes albopictus.
Moreover, the main driver for autochthonous transmission and subsequently DF outbreak, are woefully inadequate vector control interventions, such as routine monitoring of key entomological indicators and qualitative monitoring of vector control operations, weak implementation of entomological surveillance systems, insufficiently trained personnel, significant funding gaps for the prevention and control of vector-borne infections, lack of multidisciplinary and cross-sectoral collaboration and de-mobilization of resources and surveillance activities towards COVID-19 during the pandemic period. Comparable studies in Europe in Italy discovered similar drivers for autochthonous transmission.20,21
In a recent DF outbreak in Oman, the health authorities reported up to 22 May 2022, a total of 127 cases of DF of whom 58 presented to our hospital and 39 patients required hospitalization. Eighty-one percent of the patients were from Muscat governorate’s (Bawshar). Non-Omani citizens make up a large portion of the population in this district.
In this cohort, the average age was 41.0±20.0 years, while a lower age was observed in a cohort from Saudi Arabia, where the majority of affected individuals were of aged 25–44 years. Additionally, 55.2% of the affected patients in our cohort were females. Similarly, Saudi females had a higher risk rate of DF with no reported increase in dengue-related deaths.15 This finding might be related to females being exposed to mosquitos more often in households during their daily activities or being conscious of self-reporting. There may also be gender disparities in how people report illnesses or how they seek medical help.
It is critical to recognize certain factors that contribute to the severity of the illness early on while treating dengue patients. Diabetes mellitus, hypertension, coronary artery disease, and chronic obstructive pulmonary disease/bronchial asthma were more prevalent in patients with dengue shock syndrome (DSS), according to a prospective observational research in a tertiary care center in North India22 and a systematic review and meta-analysis on the prevalence of chronic comorbidities in DF.23 On the contrary, a research published in Pakistan in 2013,24 found that diabetes mellitus, hypertension, and ischemic heart disease did not increase the risk of DHF or DSS in individuals who had developed DF. In our study, 53.4% of the patients had diabetes mellitus, hypertension, and ischemic heart disease, but none had progressed to the DSS stage. This might be related to early hospitalization, prioritizing patients with comorbidities who acquire DF for specialist care and close clinical monitoring.
Thrombocytopenia has been linked to the severity of dengue infections such as DHF and DSS.25,26 Therefore, platelet counts might be utilized to predict dengue illness progression. In this cohort, all hospitalized patients with DF had good outcomes. This might be attributable to early hospital admission and prompt care at an early stage of the illness.
When compared to non-citizens, Omani citizens reported more dengue cases (86.2%) than non-citizens. This may be due to Oman residents having free access to healthcare compared to non-nationals. A similar finding was observed in Saudi Arabia.15 However, it may be also attributed to the place of residency and daily living activities.
The indigenous index case might have been exposed to infected mosquitos from an asymptomatic viremic traveler that resided in the same area/location. The risk that travelers to the endemic countries may acquire the virus and subsequently introduce it to their countries of residence exists, but further spread can be ceased and local transmission can be abrupted if surveillance and vector control interventions are taken in a timely manner. The interventions include reduction of the mosquito population, implementation of epidemiological and entomological investigations, active case finding in places of transmission, raising awareness of healthcare workers, laboratories preparedness and public education, and awareness of the preventive measures. In addition to effective vector control interventions, the dengue program should focus on detecting and predicting epidemic activity by continuous surveillance of human cases of the disease and enhancing surveillance on ports by deployment of airport fever screening systems.
Our study has a few limitations. Firstly, it is a single-center study; however, the center is the largest tertiary care facility in the country. Secondly, the sample size is small due to the small number of admitted patients. Finally, as this is a hospital-based study focusing on patients with moderate to severe disease that required hospitalization, the findings cannot be generalized to all patients with DF in the community.
Conclusion
The recent dengue outbreak in Oman is suggesting an autochthonous transmission. This highlights the urgent need for sustainable and continuing effective vector control interventions. Furthermore, a strong integrated preventative and resilient multidisciplinary control action is required crucially with sufficient resource allocation and mobilization.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We would like to thank all the participants of this study.
references
- 1. Zambrano B, San Martin JL. Epidemiology of dengue in Latin America. J Pediatric Infect Dis Soc 2014 Sep;3(3):181-182.
- 2. Global arbovirus initiative. [cited 2022 October 4]. Available from: https://www.who.int/news-room/events/detail/2022/03/31/default-calendar/global-arbovirus-initiative.
- 3. Zika and dengue among viruses that could spark the ‘next pandemic’. 2022 [cited 2022 May 20]. Available from: https://www.telegraph.co.uk/global-health/science-and-disease/zika-dengue-among-viruses-could-spark-next-pandemic/.
- 4. Dengue and severe dengue. 2022 [cited 13 May 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 5. Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 2012;6(8):e1760.
- 6. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013 Apr;496(7446):504-507.
- 7. Al-Abri SS, Kurup PJ, Al Manji A, Al Kindi H, Al Wahaibi A, Al Jardani A, et al. Control of the 2018-2019 dengue fever outbreak in Oman: a country previously without local transmission. Int J Infect Dis 2020 Jan;90:97-103.
- 8. Al Awaidy ST, Khamis F. Dengue fever: an emerging disease in Oman requiring urgent public health interventions. Oman Medical Journal 2019 Mar;34(2):91-93
- 9. Ministry of Health. Directorate General of Surveillance and Disease Control. Communicable diseases. 2017 [cited 2022 May 10]. Available from: https://www.moh.gov.om/documents/236878/0/communicable+diseases+Manual/a0577e5e-cc5a-4cb6-a460-832e37b6b587.
- Quam MB, Khan K, Sears J, Hu W, Rocklöv J, Wilder-Smith A. Estimating air travel–associated importations of dengue virus into Italy. Journal of Travel Medicine 2015 May 1;22(3):186-193.
- 11. World Health Organization. Regional Office for Europe. Climate change and communicable diseases: a manual for health workers. Copenhagen: WHO Regional Office for Europe; 2011.
- 12. Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis 2012 Jun;12(6):435-447.
- 13. Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobučar A, Pem-Novosel I, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill 2011 Mar;16(9):19805.
- 14. Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 2013 Aug;5:299-309.
- 15. Melebari S, Bakri R, Hafiz A, Qabbani F, Khogeer A, Alharthi I, et al. The epidemiology and incidence of dengue in Makkah, Saudi Arabia, during 2017-2019. Saudi Med J 2021 Nov;42(11):1173-1179.
- 16. Fan J-C, Liu Q-Y. Potential impacts of climate change on dengue fever distribution using RCP scenarios in China. Adv Clim Chang Res 2019;10(1):1-8.
- 17. Hii YL, Rocklöv J, Ng N, Tang CS, Pang FY, Sauerborn R. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Glob Health Action 2009 Nov;2(1):2036.
- 18. Alto BW, Bettinardi D. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am J Trop Med Hyg 2013 Mar;88(3):497-505.
- 19. Gui H, Gwee S, Koh J, Pang J. Weather factors associated with reduced risk of dengue transmission in an urbanized tropical city. Int J Environ Res Public Health 2021 Dec;19(1):339.
- 20. Jourdain F, Roiz D, de Valk H, Noël H, L’Ambert G, Franke F, et al. From importation to autochthonous transmission: drivers of chikungunya and dengue emergence in a temperate area. PLoS Negl Trop Dis 2020 May;14(5):e0008320.
- 21. Barzon L, Gobbi F, Capelli G, Montarsi F, Martini S, Riccetti S, et al. Autochthonous dengue outbreak in Italy 2020: clinical, virological and entomological findings. J Travel Med 2021 Dec;28(8):taab130.
- 22. Jain S, Mittal A, Sharma SK, Upadhyay AD, Pandey RM, Sinha S, et al. Predictors of dengue-related mortality and disease severity in a tertiary care center in north India. Open Forum Infect Dis 2017 May 5;4(2):ofx056.
- 23. Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, et al. Prevalence of chronic comorbidities in dengue fever and West Nile virus: a systematic review and meta-analysis. PLoS One 2018 Jul;13(7):e0200200.
- 24. Mahmood S, Hafeez S, Nabeel H, Zahra U, Nazeer H. Does comorbidity increase the risk of dengue hemorrhagic fever and dengue shock syndrome? International Scholarly Research Notices 2013;2013.
- 25. Kularatnam GA, Jasinge E, Gunasena S, Samaranayake D, Senanayake MP, Wickramasinghe VP. Evaluation of biochemical and haematological changes in dengue fever and dengue hemorrhagic fever in Sri Lankan children: a prospective follow up study. BMC Pediatr 2019 Apr;19(1):87.
- 26. Lam PK, Ngoc TV, Thu Thuy TT, Hong Van NT, Nhu Thuy TT, Hoai Tam DT, et al. The value of daily platelet counts for predicting dengue shock syndrome: results from a prospective observational study of 2301 Vietnamese children with dengue. PLoS Negl Trop Dis 2017 Apr;11(4):e0005498.