According to the Global Cancer Observatory, breast cancer comprises approximately 25% of all cancers in women and was ranked in 2020 as the cancer with the highest incidence, prevalence, and mortality.1
The causative risk factors for breast cancer are broadly classified as modifiable and nonmodifiable. Genetic disposition is the prime nonmodifiable risk factor. Women with mutations in the BRCA1 and BRCA2 genes have a higher risk for breast cancer in the postmenopausal period.2 In these women, if the menopause phase is acquired with a long onset, the consequent longer-term estrogen exposure might also be a risk factor. Family history with one or both factors increases the overall risk of breast cancer.3
Meanwhile, modifiable risk factors of breast cancer are diverse and often lifestyle-related, such as obesity, excessive alcohol consumption, hormone replacement therapy, and the use of certain drugs.4
Among the drugs under the scanner are antipsychotics which are often used together with antidepressants by the Asian geriatric population.5 Excessive use of antipsychotics has been suggested to be a risk factor for breast cancer through hormonal modulation. The rising tendency to overprescribe antipsychotics (often without valid diagnosis of mental illness) is reported to have led to their overconsumption.6
Antipsychotics function as dopamine antagonists in the body through post-synaptic D2 receptor blockade in the pituitary gland. Dopamine is a natural inhibitor of prolactin, a hormone that regulates breast development and several physiological functions. Reduced dopamine availability in the system increases the levels of plasma prolactin, causing lactotrophic cells to become more active than normal, raising the risk of breast cancer.7
The question of whether antipsychotics increase the risk of breast cancer is still not settled. While several studies reported a significant correlation between antipsychotic drug use and breast cancer risk,8–10 others found it non-significant.11–13 Therefore, we launched the current systematic review and meta-analysis to evaluate the relationship, if any, between antipsychotic drugs and breast cancer incidence.
Methods
We searched medical databases such as PubMed, ScienceDirect, Cochrane, Medline, and other appropriate sources for research papers containing the keywords ‘antipsychotic’, ‘breast cancer’, and ‘risk.’ Google Scholar was excluded to avoid grey literature.
The inclusion criteria were the following: (1) the study should report an association between the use of antipsychotic agents and breast cancer incidence in a patient group; (2) the study should report the relative risk of breast cancer among patient groups both qualitatively and quantitatively using the odds ratio (OR) with 95% CI; (3) the study should have a non-randomized controlled design, either case-control or cohort; and (4) the study should be published in either English or Indonesian language.
Two authors independently conducted the study selection, quality assessment, and data extraction. Differences of opinion between them were resolved through consideration and discussion with the third author. All protocols in this study’s writing were developed based on the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [Figure 1].14
 Figure 1: Diagram depicting the selection process of the study.
Figure 1: Diagram depicting the selection process of the study.
For the quality assessment of the selected studies, we used the Newcastle-Ottawa Scale adaptation. To evaluate the case-control studies, we used the following assessment criteria, with maximum points given in parentheses: selection (4 points), comparability (2 points), and exposure (2 points). For cohort studies, the factors assessed included selection (4 points), comparability (1 point), and outcomes (3 points). The total points earned ranged from 0 indicating the worst quality to 8 indicating the best quality. Based on the above, each paper was classified as good (total points > 7), moderate (5–6), or poor (< 4).
Two authors independently extracted data on baseline characteristics, exposures, and outcomes from the selected studies. Also extracted were the author identity, publication year, study design, country, and the number of participants in both the case and control groups. Studies without sufficient data were excluded from the quantitative analysis (meta-analysis).
The main intended outcome of this systematic review was the estimation of the risk of breast cancer from the use of antipsychotic drugs. We employed the OR parameter with a 95% CI using a random effect model in view of the expected diversities in the numbers and characteristics of participants between different studies. The heterogeneity level was assessed using the Q-test with a significance value at p < 0.10 and an assessment of the I2 statistic. The higher significance (p-value) threshold was given because the statistical tests for heterogeneity were not particularly potent. Quantitative analysis of each group of antipsychotic drugs was conducted to determine the OR for each sub-group.
Results
The literature search process is depicted in the PRISMA flow diagram [Figure 1]. The initial search yielded 890 studies, and manual review yielded three more. After excluding duplicates and screening titles and abstracts, 20 studies were shortlisted for feasibility assessment, nine of which were excluded. Finally n = 11 studies were chosen to be eligible for qualitative synthesis out of which five were also suitable for meta-analysis.
All the 11 selected studies were written in English. Five were case-control type and six were cohort type. The studies emerged from five countries: USA (four studies), UK (two), Denmark (two), Taiwan (two), and Sweden (one). The cohort sizes (including controls) ranged from 256 to 663 960, with a total of 1 419 997 participants. The number of breast cancer patients per study ranged from 91 to 60 360 with an overall total of 97 566. Five studies classified antipsychotic use into two types: typical (first generation) and atypical (second generation). Most studies were retrospective, based on past patient data from medical records. The study quality ranged from 5 points (moderate) to 8 points (high) with an average of 6.7 points, indicating that the overall quality was suitable for meta-analysis. The summaries of the included studies are presented in Table 1.
Table 1: The characteristics and results of the 11 studies that evaluated breast cancer risks of antipsychotics.
|
Kanhouwa,15 1984
|
121
|
65 555
|
US
|
Case-control
|
Neuroleptic
|
Neuroleptics had no significant effect on breast cancer.
|
Moderate
|
|
Kelly,16 1999
|
91
|
256
|
US
|
Case-control
|
Neuroleptic
|
There was no significantly increased risk of breast cancer.
|
Good
|
|
Wang,8 2002
|
2467
|
52 819
|
US
|
Cohort
|
Dopamine antagonist
|
Antipsychotic dopamine antagonists could slightly increase (with statistical significance) the risk of breast cancer.
|
Good
|
|
Dalton,17 2006
|
341
|
25 264
|
Denmark
|
Cohort
|
Neuroleptic
|
There was no increased risk of breast cancer.
|
Good
|
|
Hippisley-Cox,9 2007
|
1478
|
8121
|
UK
|
Nested case-control
|
Conventional antipsychotics, atypical antipsychotics, and lithium
|
There was an increased risk of breast cancer in schizophrenics.
|
Moderate
|
|
Azoulay,18 2011
|
1237
|
106 362
|
UK
|
Nested case-control
|
Atypical and typical antipsychotics
|
When compared with typical antipsychotics, atypical antipsychotics did not appear to increase the risk of breast cancer.
|
Moderate
|
|
Reutfors,19 2017
|
348
|
55 976
|
Sweden
|
Cohort
|
Risperidone, other atypical antipsychotics, and typical antipsychotics
|
Risperidone use did not increase the short-term risk of breast cancer compared with other antipsychotic agents.
|
Good
|
|
Chou,10 2017
|
29 641
|
88 923
|
Taiwan
|
Cohort
|
First-generation antipsychotics (FGA), risperidone, paliperidone, amisulpride, and other second-generation antipsychotics (SGA)
|
Schizophrenic patients who consumed both FGA and SGA had slightly higher risk of breast cancer than non-schizophrenic patients.
|
Good
|
|
Pottegard,11 2018
|
60 360
|
663 960
|
Denmark
|
Case-control
|
Single antipsychotic
|
Overall, there was no clinically important association between antipsychotic use and breast cancer risk.
|
Moderate
|
|
Tsai,13 2018
|
1449
|
233 237
|
Taiwan
|
Cohort
|
Risperidone, other atypical antipsychotics, and conventional antipsychotics.
|
There was no evidence of an increased risk of breast cancer associated with risperidone compared to other atypical or conventional antipsychotics.
|
Good
|
*The number of patients who took antipsychotics and developed breast cancer.
**The total number of patients in the study.
 Figure 2: Forest plot regarding the overall use of antipsychotic drugs against breast cancer.
Figure 2: Forest plot regarding the overall use of antipsychotic drugs against breast cancer.
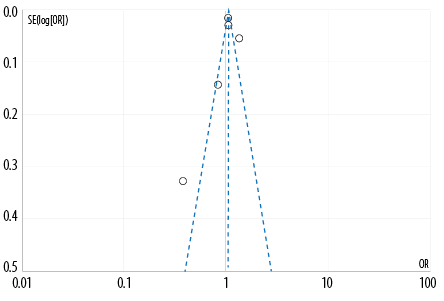 Figure 3: Funnel plot regarding the overall use of antipsychotic drugs against breast cancer. (Each circle represents one study.)
Figure 3: Funnel plot regarding the overall use of antipsychotic drugs against breast cancer. (Each circle represents one study.)
Meta-analysis (quantitative synthesis) of five studies (9–11, 15, 16) with 81 766 breast cancer patients and 1 150 316 controls demonstrated a lack of significant association between the overall use of antipsychotics and breast cancer incidence (OR = 1.06, 95% CI: 0.94–1.19; p = 0.36) [Figure 2]. The relationships of distinct subgroups of antipsychotic drugs on breast cancer could not be analyzed due to inadequate subgroup-wise data.
The heterogeneity of the studies was assessed using the Q-test. The results of the analysis show evidence of heterogeneity (p < 0.10) in the overall analysis. The I2 statistical assessment also demonstrates heterogeneity (I2 > 75%). Therefore, this finding supports using a random effect model to determine the correlation and effect estimation on data synthesis. The risk of publication bias was evaluated using the Egger's test and funnel plot symmetry assessment. The Egger's test revealed significant publication bias in the results of quantitative analysis (p < 0.05). The funnel plot [Figure 3] indicates a small possibility of publication bias.
Discussion
Typical antipsychotic drugs, especially phenothiazines, have antiproliferative effects on the breast cancer MCF-7 cell line. Among the phenothiazines, perphenazine and chlorpromazine have the best cytotoxic activity,20 though the pharmacological mechanism remains unclear. The result of cytotoxicity may not be associated with ligand-based antipsychotics at dopamine and serotonin receptors because the concentrations required to induce cytotoxicity are many times greater than those required to satisfy these receptors.21
Antipsychotics may be inducing cytotoxicity to cancer cells by chemically interacting with the cell membrane. This may be facilitated by the drug’s amphiphilic properties, thereby upsetting the cell’s cholesterol homeostasis, leading to its apoptosis.22 In addition, phenothiazines at pharmacological doses are known to increase the body’s natural killer cell activity, mitogen-induced activation, and human T-cell proliferation. Thus, the ability of phenothiazines to stimulate the immune system may boost the natural resistance against tumor cells.20
Meanwhile, atypical antipsychotics such as clozapine with its main metabolite N-desmethylclozapine, have toxic effects on normal cells on myeloid maturation and myeloid mitotic components. This effect is implicated in agranulocytosis which occurs in approximately 1% of patients treated with clozapine.20
Another atypical antipsychotic, sertindole, is known to cause cell apoptosis by directly inducing autophagy and inhibiting 5-HT6 receptors. The administration of sertindole near the maximum therapeutic dose weakens breast tumor growth by 22.7% in xenotransplant mice. However, the mechanism of this inhibition remains unclear. The most likely explanation is the inhibition of the dopamine and serotonin receptors.23
Several previous studies have also demonstrated antipsychotic drugs as having no significant association with the incidence of breast cancer in both men and women, thus supporting our findings.24,25 On the other hand, in-vitro and in-vivo studies on antipsychotic drugs such as the pimozide, chlorpromazine, and piperidine classes have indicated their therapeutic potential for several types of cancer including breast cancer.26–28 Besides, atypical antipsychotic drugs such as olanzapine are known to reduce cancer-related symptoms and side effects of chemotherapy such as nausea and vomiting.29 However, others such as clozapine in conjunction with chemotherapy might increase cancer-related complications such as neutropenia.30
Despite our comprehensive literature search, only a few studies of sufficient specificity and quality could be identified. Even these had insufficient data on the antipsychotic subclasses. Therefore our meta-analysis could not evaluate the impact of individual antipsychotic subclasses on breast cancer.
Conclusion
This systematic review and meta-analysis found that antipsychotic drug usage did not significantly increase breast cancer risk. Further studies are needed regarding the relationship between subclass-wise antipsychotic usage and breast cancer risk in the long term. More randomized controlled trials, cohort, or case-control studies need to be conducted on the relationship between the usage of various antipsychotic subclasses and the risk of developing breast cancer.
Based on such studies, updated information on each antipsychotic subclass and their risk potential for breast cancer should be made available to clinicians.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021 May;71(3):209-249.
- 2. Hilgart JS, Coles B, Iredale R. Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database Syst Rev 2012 Feb;2012(2):CD003721.
- 3. Surakasula A, Nagarjunapu GC, Raghavaiah KV. A comparative study of pre- and post-menopausal breast cancer: risk factors, presentation, characteristics and management. J Res Pharm Pract 2014 Jan;3(1):12-18.
- 4. Li J, Humphreys K, Ho PJ, Eriksson M, Darai-Ramqvist E, Lindström LS, et al. Family history, reproductive, and lifestyle risk factors for fibroadenoma and breast cancer. JNCI Cancer Spectr 2018 Dec;2(3):pky051.
- 5. Dong M, Zeng LN, Zhang Q, Ungvari GS, Ng CH, Chiu HF, et al. Concurrent antipsychotic use in older adults treated with antidepressants in Asia. Psychogeriatrics 2019 Jul;19(4):333-339.
- 6. Correll CU, Blader JC. Antipsychotic use in youth without psychosis a double-edged sword. JAMA Psychiatry 2015 Sep;72(9):859-860.
- 7. Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 2014 May;28(5):421-453.
- 8. Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, et al. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 2002 Dec;59(12):1147-1154.
- 9. Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry 2007 Dec;64(12):1368-1376.
- 10. Wu Chou AI, Wang YC, Lin CL, Kao CH. Female schizophrenia patients and risk of breast cancer: a population-based cohort study. Schizophr Res 2017 Oct;188:165-171.
- 11. Pottegård A, Lash TL, Cronin-Fenton D, Ahern TP, Damkier P. Use of antipsychotics and risk of breast cancer: a Danish nationwide case-control study. Br J Clin Pharmacol 2018 Sep;84(9):2152-2161.
- 12. George A. Antipsychotic drug use and postmenopausal breast cancer risk in the women’s health initiative (WHI): a prospective cohort study [thesis]. University of Massachusetts Amherst; 2019.
- 13. Tsai K-Y, Wu H-C, Shen S-P, Qiu H, Wang Y, Pai H, et al. Risperidone exposure and breast cancer risk: a cohort study using the Taiwan national health insurance research database. Neuropsychiatry (London) 2018; 8(5):1549-1558.
- 14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009 Jul;339(1):b2700.
- 15. Kanhouwa S, Gowdy JM, Solomon JD. Phenothiazines and breast cancer. journal of the National Medical Association 1984;76(8):785.
- 16. Kelly JP, Rosenberg L, Palmer JR, Rao RS, Strom BL, Stolley PD, et al. Risk of breast cancer according to use of antidepressants, phenothiazines, and antihistamines. Am J Epidemiol 1999 Oct;150(8):861-868.
- 17. Dalton SO, Johansen C, Poulsen AH, Nørgaard M, Sørensen HT, McLaughlin JK, et al. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br J Cancer 2006 Oct;95(7):934-939.
- 18. Azoulay L, Yin H, Renoux C, Suissa S. The use of atypical antipsychotics and the risk of breast cancer. Breast Cancer Res Treat 2011 Sep;129(2):541-548.
- 19. Reutfors J, Wingård L, Brandt L, Wang Y, Qiu H, Kieler H, et al. Risk of breast cancer in risperidone users: a nationwide cohort study. Schizophr Res 2017 Apr;182:98-103.
- 20. Nordenberg J, Fenig E, Landau M, Weizman R, Weizman A. Effects of psychotropic drugs on cell proliferation and differentiation. Biochemical Pharmacology 1999;58(8):1229-1236.
- 21. Donohue JM, Frank RG. Estimating medicare part D’s impact on medication access among dually eligible beneficiaries with mental disorders. Psychiatr Serv 2007 Oct;58(10):1285-1291.
- 22. Wiklund ED, Catts VS, Catts SV, Ng TF, Whitaker NJ, Brown AJ, et al. Cytotoxic effects of antipsychotic drugs implicate cholesterol homeostasis as a novel chemotherapeutic target. Int J Cancer 2010 Jan;126(1):28-40.
- 23. Zhang W, Zhang C, Liu F, Mao Y, Xu W, Fan T, et al. Antiproliferative activities of the second-generation antipsychotic drug sertindole against breast cancers with a potential application for treatment of breast-to-brain metastases. Sci Rep 2018 Oct;8(1):15753.
- 24. De Hert M, Peuskens J, Sabbe T, Mitchell AJ, Stubbs B, Neven P, et al. Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand 2016 Jan;133(1):5-22.
- 25. George A, Sturgeon SR, Hankinson SE, Shadyab AH, Wallace RB, Reeves KW. Psychotropic medication use and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 2020 Jan;29(1):254-256.
- 26. Yang CE, Lee WY, Cheng HW, Chung CH, Mi FL, Lin CW. The antipsychotic chlorpromazine suppresses YAP signaling, stemness properties, and drug resistance in breast cancer cells. Chem Biol Interact 2019 Apr;302:28-35.
- 27. Dakir H, Pickard A, Srivastava K, McCrudden CM, Gross SR, Lloyd S, et al. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget 2018 Oct;9(79):34889-34910.
- 28. Shaw V, Srivastava S, Srivastava SK. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin Cancer Biol 2021 Jan;68(1):75-83.
- 29. Sutherland A, Naessens K, Plugge E, Ware L, Head K, Burton MJ, et al. Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database Syst Rev 2018 Sep;9(9):CD012555.
- 30. Grainger BT, Arcasoy MO, Kenedi CA. Feasibility of myelosuppressive chemotherapy in psychiatric patients on clozapine: a systematic review of the literature. Eur J Haematol 2019 Oct;103(4):277-286.