Hepatitis C virus (HCV), a single-stranded RNA virus in the family Flaviviridae, is a major cause of mortality in chronic hemodialysis patients.1 Due to impaired immunity associated with renal disease and frequent medical interventions, hemodialysis patients are at increased risk of HCV infection, which may result in cardiovascular disease and liver failure in the long term.2 The higher prevalence of HCV infection among hemodialysis patients can be explained by exposure to infected blood and contaminated equipment during dialysis treatment.3,4 Patient-to-patient nosocomial transmission of the virus in multiple bedded hemodialysis units is also suggested as a secondary possibility.5
The global prevalence of chronic kidney disease is on the rise due to the increasing rates of high blood pressure and diabetes mellitus.2,6 Early detection and treatment of HCV-infected hemodialysis patients can improve survival rates.2 Considering the asymptomatic nature of chronic HCV infection and the fact that most infected patients are not aware of their infection, routine screening of hemodialysis patients for HCV markers is of great importance. Moreover, preventive strategies are needed to minimize the risk of HCV transmission in hemodialysis centers. This can be achieved through implementing standard precautions and biosafety measures in the dialysis units, training the medical staff, and educating the patients on the virus transmission routes and possible preventive measures from their side.2,3
The prevalence and epidemiology of HCV infection vary across countries. To evolve better strategies for the prevention and management of HCV infection, updated epidemiological data is essential. In Iran, such data is incomplete. For example, an HCV prevalence of 3.36% has been reported among hemodialysis patients in the Hormozgan province of southern Iran.7 However, there is no data from its neighboring province, Bushehr. Therefore, this study was designed to investigate the prevalence, genotype distribution, and risk factors of HCV infection among patients on regular hemodialysis in Bushehr province. Our results are expected to help fill the research gap in the epidemiological information regarding this vulnerable patient group in Bushehr, and share our results with the health policymakers.
Methods
All chronic hemodialysis patients attending the hemodialysis centers of the Bushehr University of Medical Sciences for regular hemodialysis during the year 2020 were invited to participate in this descriptive-analytical cross-sectional study. Patients on occasional hemodialysis were excluded from the study. A total of 279 patients on regular hemodialysis (151 males; 128 females) living in the cities of Dashtestan, Genaveh, and Bushehr participated. The control group comprised 277 non-patient volunteers who did not have history of hemodialysis, receiving blood transfusion, or injecting illicit drugs. The controls were matched with the subjects in respect of the date of participation, sex, and age (± 3 years). At the time of interviewing, a questionnaire was used to collect demographic and clinical information from the participants and controls. All participants and controls signed written informed consent forms to use their serum samples for HCV detection and analysis. The study was approved by the ethics committee of the Bushehr University of Medical Sciences with research project number IR.BPUMS.REC.1395.184.
Fourth-generation enzyme-linked immunosorbent assay was used to detect anti-HCV antibodies in the serum samples of the participants (HCV Ab ELISA kit, DIA.PRO, Milan, Italy) according to the manufacturer’s instructions. The specificity and sensitivity of this kit are 99.5% and 100%, respectively.
The molecular detection of HCV infection was performed by semi-nested reverse transcriptase-polymerase chain reaction (RT-PCR) assay, targeting 5’ untranslated region (5’UTR) and core region of the genome, and sequencing as described in our previous studies.8,9 HCV RNA was extracted from the serum samples of the participants using High Pure Viral Nucleic Acid Kit (Roche, Germany) and reverse-transcription into cDNA using the SuperScript III cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Thereafter, the 680 bp and 580 bp length fragments of HCV genome were amplified using outer primers [forward primer: AGCGTCTAGCCATGGCGT (-268 to -251); reverse primer: ATGTACCCCATGAGGTCGGC (+410 to +391)] and inner primers [forward primer: GTGGTCTGCGGAACCGG (-199 to -183); reverse primer: CACGTTAGGGTATCGATGAC (+383 to +364)]. The PCR reaction was used in a final volume of 50 µL and included 5 µL 10X PCR buffer containing 1.5 mM MgCl2, 200 µM of each dNTP, 20 pM of each primer, and 2U Taq DNA polymerase (Roche, Germany). Moreover, 5 µL of template cDNA and 0.5 µL of the first-round PCR product were used as templates for the first and second rounds of the nested PCR, respectively. The PCR condition was as follows: initial denaturation at 95 °C for 5 min, followed by denaturation at 95 °C for 30 sec, annealing at 60 °C for 30 sec, extension at 72 °C for 30 sec, and a final extension at 72 °C for 5 min. The first and second rounds of nested PCR were conducted for 25 and 35 cycles, respectively. The sequences of primers and regions in the genome for detection of HCV are summarized in Table 1. The 580 bp length fragments were sequenced to determine HCV genotypes (Macrogen Co., Korea). Briefly, the isolated sequences were aligned and compared with the reference sequences representing the standard genotypes of HCV available at the nucleotide database of the NCBI by the ClustalW program in the MEGA software version 7.0 (Biodesign Institute, Tempe, AZ, USA).
Table 1: Sequences of primers for amplification of 5’UTR and core region of HCV genome.
|
8,9
|
680 bp
|
-268 to -251
|
5'UTR
|
AGCGTCTAGCCATGGCGT
|
g-HCVF1
|
|
|
410 to 391
|
Core
|
ATGTACCCCATGAGGTCGGC
|
S-HCR1
|
|
8,9
|
580 bp
|
-199 to -183
|
5'UTR
|
GTGGTCTGCGGAACCGG
|
g-HCVF2
|
UTR: untranslated region; HCV: hepatitis C virus.
SPSS statistical software (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.) was used for data analyses, and p-values < 0.05 were considered significant. Data was subjected to Student’s t-test, χ2 test, and Fisher exact test as applicable. Logistic regression analysis was used to identify the risk factors associated with HCV prevalence among hemodialysis patients, and odds ratio with 95% CI was calculated.
Results
Our subjects comprised 279 patients who were on regular hemodialysis, of whom 176 were from Bushehr, 57 from Dashtestan, and 46 from Genaveh. The mean age±SD of these patients was 59.9±14.2 (range = 21–87 years). Of the 279 hemodialysis patients, 15 (5.4%, 95%CI: 3.30–8.68) tested positive for anti-HCV antibodies (6.0% of men and 4.7% of women). The prevalence of anti-HCV antibodies in the control group (n = 277) was 1.1% (95%CI: 0.39–3.12). Thus, the hemodialysis patients had significantly higher seroprevalence of HCV than the controls (p = 0.007). Anti-HCV seropositivity was also significantly associated with ethnicity so Arab hemodialysis patients (17.4%; 95%CI: 6.98–37.14) endorsing higher anti-HCV seroprevalence than those with Fars ethnicity (4.3%; p = 0.026). Three-quarters of patients with Arab ethnicity had hemodialysis duration of more than four years which explains the higher prevalence among them because the more dialysis sessions one has had, the higher the risk of HCV infection. Notably, all of the anti-HCV seropositive hemodialysis patients had normal levels of liver enzymes and were negative for HIV and hepatitis B virus.
The anti-HCV seropositivity was higher among the hemodialysis patients aged < 60 years (6.0%) compared to the hemodialysis patients aged > 60 years (4.0%). However, this difference was statistically insignificant (p = 0.776). Overall, anti-HCV seropositive hemodialysis patients had a lower mean age (56.0±15.1) compared to anti-HCV seronegative hemodialysis patients (60.1±14.2), again without significance (p = 0.278). The anti-HCV prevalence was higher among male hemodialysis patients (6.0%), residents of Genaveh (6.5%), patients who were on regular hemodialysis for < 1 year (11.6%), and patients with upper diplomas (7.1%). Nevertheless, anti-HCV prevalence among hemodialysis patients was not significantly associated with their sex, duration of hemodialysis, history of blood transfusion, level of education, or place of residence [Table 2].
Table 2: Prevalence of anti-HCV antibodies according to socio-demographic and qualitative variables among hemodialysis patients in Bushehr province.
|
Age groups, years
|
|
|
|
0.776
|
|
≤ 39
|
28 (10.0)
|
26 (92.9)
|
2 (7.1)
|
|
|
40–49
|
27 (9.7)
|
25 (92.6)
|
2 (7.4)
|
|
|
50–59
|
68 (24.4)
|
63 (92.6)
|
5 (7.4)
|
|
|
60–69
|
83 (29.7)
|
80 (96.4)
|
3 (3.6)
|
|
|
70–79
|
54 (19.4)
|
51 (94.4)
|
3 (5.6)
|
|
|
≥ 80
|
19 (6.8)
|
19 (100.0)
|
0 (0.0)
|
|
|
Sex
|
|
|
|
0.422
|
|
Female
|
128 (45.9)
|
122 (95.3)
|
6 (4.7)
|
|
|
Male
|
151 (54.1)
|
142 (94.0)
|
9 (6.0)
|
|
|
Place of residence
|
|
|
|
0.396
|
|
Bushehr
|
176 (63.1)
|
165 (93.8)
|
11 (6.3)
|
|
|
Dashtestan
|
57 (20.4)
|
56 (98.2)
|
1 (1.8)
|
|
|
Genaveh
|
46 (16.5)
|
43 (93.5)
|
3 (6.5)
|
|
|
Ethnicity
|
|
|
|
0.026
|
|
Fars
|
256 (91.8)
|
245 (95.7)
|
11 (4.3)
|
|
|
Arab
|
23 (8.2)
|
19 (82.6)
|
4 (17.4)
|
|
|
Duration of hemodialysis, years
|
|
|
0.237
|
|
< 1
|
43 (15.4)
|
38 (88.4)
|
5 (11.6)
|
|
|
1–2
|
105 (37.6)
|
101 (96.2)
|
4 (3.8)
|
|
|
3–4
|
58 (20.8)
|
56 (96.6)
|
2 (3.4)
|
|
|
> 4
|
73 (26.2)
|
69 (94.5)
|
4 (5.5)
|
|
|
Education
|
|
|
|
0.943
|
|
Under diploma
|
231 (82.8)
|
219 (94.8)
|
12 (5.2)
|
|
|
Diploma
|
34 (12.2)
|
32 (94.1)
|
2 (5.9)
|
|
|
Upper diploma
|
14 (5.0)
|
13 (92.9)
|
1 (7.1)
|
|
|
History of blood transfusion
|
|
|
0.924
|
|
No
|
35 (12.5)
|
33 (94.3)
|
2 (5.7)
|
|
HCV: hepatitis C virus; HCV Ab: HCV antibodies.
Molecular evaluation revealed that two hemodialysis patients (0.7%, 95%CI: 0.22–2.55) had HCV viremia with genotype 3a. They were 43- and 45-year-old men with normal levels of alanine aminotransferase and aspartate aminotransferase who were under regular hemodialysis for more than four years [Table 3]. These cases were positive in the first and second rounds of amplification. In addition, one of the controls was positive for HCV RNA (0.4%, 95%CI: 0.08–1.98).
Table 3: Prevalence of HCV viremia according to socio-demographic and qualitative variables among hemodialysis patients in Bushehr province.
|
Age groups, years
|
|
|
|
0.002
|
|
≤ 39
|
28 (10.0)
|
28 (100)
|
0 (0.0)
|
|
|
40–49
|
27 (9.7)
|
25 (92.6)
|
2 (7.4)
|
|
|
50–59
|
68 (24.4)
|
68 (100)
|
0 (0.0)
|
|
|
60–69
|
83 (29.7)
|
83 (100)
|
0 (0.0)
|
|
|
70–79
|
54 (19.4)
|
54 (100)
|
0 (0.0)
|
|
|
≥ 80
|
19 (6.8)
|
19 (100)
|
0 (0.0)
|
|
|
Sex
|
|
|
|
0.292
|
|
Female
|
128 (45.9)
|
128 (100)
|
0 (0.0)
|
|
|
Male
|
151 (54.1)
|
149 (98.7)
|
2 (1.3)
|
|
|
City of residence
|
|
|
|
0.555
|
|
Bushehr
|
176 (63.1)
|
174 (98.9)
|
2 (1.1)
|
|
|
Dashtestan
|
57 (20.4)
|
57 (100)
|
0 (0.0)
|
|
|
Genaveh
|
46 (16.5)
|
46 (100)
|
0 (0.0)
|
|
|
Ethnicity
|
|
|
|
0.158
|
|
Fars
|
256 (91.8)
|
255 (99.6)
|
1 (0.4)
|
|
|
Arab
|
23 (8.2)
|
22 (95.7)
|
1 (4.3)
|
|
|
Duration of hemodialysis, years
|
|
|
0.128
|
|
< 1
|
43 (15.4)
|
43 (100)
|
0 (0.0)
|
|
|
1–2
|
105 (37.6)
|
105 (100)
|
0 (0.0)
|
|
|
3–4
|
58 (20.8)
|
58 (100)
|
0 (0.0)
|
|
|
> 4
|
73 (26.2)
|
71 (97.3)
|
2 (2.7)
|
|
|
Education
|
|
|
|
0.811
|
|
Under diploma
|
231 (82.8)
|
229 (99.1)
|
2 (0.9)
|
|
|
Diploma
|
34 (12.2)
|
34 (100)
|
0 (0.0)
|
|
HCV RNA: hepatitis C virus ribonucleic acid.
Although our hemodialysis cases had a higher prevalence of HCV viremia than the non-hemodialysis controls, the difference was not statistically significant (p = 0.503). The mean age of hemodialysis patients with HCV viremia (44.0±1.4) was lower than that of HCV RNA-negative hemodialysis patients (60.0±14.2), but this difference was statistically insignificant (p = 0.114). Electrophoresis of semi-nested RT-PCR products of the 5’UTR and core region of HCV genome extracted from serum samples of hemodialysis patients on 2% agarose gel is shown in Figures 1 and 2.
 L: 100-bp DNA ladder; p: positive control; N: negative control; 4: amplified product (680 bp) on 2% agarose gel electrophoresis; RT-PCR: reverse transcription polymerase chain reaction; HCV: hepatitis C virus.
L: 100-bp DNA ladder; p: positive control; N: negative control; 4: amplified product (680 bp) on 2% agarose gel electrophoresis; RT-PCR: reverse transcription polymerase chain reaction; HCV: hepatitis C virus.
Figure 1: The first round of semi-nested RT-PCR amplification of HCV RNA extracted from samples of hemodialysis patients.
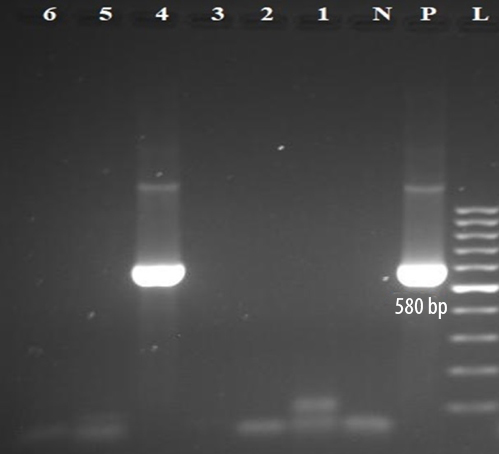 L: 100-bp DNA ladder; p: positive control; N: negative control; 4: amplified product (580 bp) on 2% agarose gel electrophoresis; RT-PCR: reverse transcription polymerase chain reaction; HCV: hepatitis C virus.
L: 100-bp DNA ladder; p: positive control; N: negative control; 4: amplified product (580 bp) on 2% agarose gel electrophoresis; RT-PCR: reverse transcription polymerase chain reaction; HCV: hepatitis C virus.
Figure 2: The second round of semi-nested RT-PCR amplification of HCV RNA extracted from samples of hemodialysis patients.
Discussion
This study is the first one that has determined the prevalence and genotypic pattern of HCV infection among patients on regular hemodialysis in Bushehr. According to our results, anti-HCV seroprevalence was more frequent in hemodialysis patients (5.4%) than in non-hemodialysis controls (1.1%) and blood donors (0.1%) living in this region.10 Moreover, hemodialysis patients had a higher seroprevalence of HCV than blood donors (0.5%) and the general population (0.6%) of Iran.11,12 This suggests that nosocomial transmission of infection is probably the main route of HCV transmission among hemodialysis patients in Bushehr. In addition, since hemodialysis patients often suffer from anemia and with their history of blood transfusion, there is a possibility of transmission via blood transfusion.4,13 Therefore, the screening process of donated blood should be thoroughly reevaluated.
The prevalence of 5.4% of anti-HCV antibodies in hemodialysis patients in Bushehr detected in this study is lower than the range of 7–38% reported from other parts of Iran.14 It is also far below the overall seroprevalence of HCV among hemodialysis patients in Iran (12%) and the Middle East (25.3%).15,16 It is also lower than those reported among hemodialysis patients of the neighboring countries: 7.1% in Iraq,17 19% in Turkey,18 32.33% in Pakistan,19 and 46.7% in Saudi Arabia,20 but higher than the seroprevalence rates in Australia (2.3%),21 Colombia (2.9%),22 and Somalia (3.2%).23 These variations across countries could be due to differences in the epidemiology of HCV infection, overall rates of HCV endemicity, risk factors, and variations in biosafety practices in dialysis units in different countries and geographical regions. However, differences in sensitivity and specificity of diagnostic assays, in addition to the socio-demographic and ethnic variations between countries and populations within a country may also partly explain these observed variations. In the current cohort, anti-HCV seropositivity was also significantly associated with ethnicity, so ethnically Arab hemodialysis patients had significantly higher anti-HCV seroprevalence compared to Fars patients (p = 0.026). In fact, except for ethnicity, no significant risk factor was found for the seroprevalence of HCV among hemodialysis patients in Bushehr.
In this study, the anti-HCV antibody prevalence was higher among male hemodialysis patients, residents of Genaveh, patients who were less than one year on regular hemodialysis, and upper diploma holders; however, these findings were not statistically significant. Another non-significant finding was the fall in the rates of seropositivity with age, with lower prevalence of anti-HCV antibodies among patients aged > 60 years. The probable reason might be that the older hemodialysis patients were infected by HCV in previous years; after recovery, their antibody titer decreased over time and reached undetectable levels. Although 87.5% of hemodialysis patients had received packed red blood cell, there was no significant association between their HCV seroprevalence and history of blood transfusion (p = 0.924).
According to the molecular evaluation, HCV RNA was detected in 0.7% of hemodialysis patients by semi-nested RT-PCR. HCV viremia was more prevalent in our hemodialysis patients (0.7%) than in non-hemodialysis controls (0.4%) and the general population of Iran (0.4%).12 The viremic cases were in the age group 40–49 years and had normal levels of liver enzymes. The diagnostic values of serum aminotransferases tend to be insignificant in patients on maintenance hemodialysis. The presence of ultraviolet light-absorbing components and uremic toxins in the blood as well as vitamin B6 deficiency could reduce the transaminase levels in the samples of HCV-infected patients who are on long-term dialysis.1,24 Therefore, HCV infection among hemodialysis patients might remain undiagnosed due to the asymptomatic nature of chronic HCV infection and normal ranges of liver enzymes.25 Considering the fact that the level of HCV RNA may get reduced during the process of hemodialysis, the sample to detect HCV RNA should be collected before the dialysis session.1 Moreover, HCV genotype 3a we found is similar to the predominant genotype in this region8,9,26 and is prevalent among injecting drug users, who are the significant drivers of HCV transmission in the community.27
The association between HCV infection and renal injury is well-defined. The action of HCV in the development of kidney disorders could be through overstimulation of B lymphocytes in response to persistent HCV infection and production of mixed cryoglobulinemia which are involved in the membranoproliferative glomerulonephritis,5,24 or direct effects of HCV-related proteins on the mesangium leading to mesangial injury and subsequently proteinuria.24 There is also the risk of increased exposure to HCV during end-stage renal disease due to frequent medical interventions, prolonged vascular access, and contaminated equipment.5 On the one hand we found higher rates of anti-HCV seropositivity among patients with less than one-year history of regular hemodialysis, which may support the idea that HCV probably has a role in the development of kidney disorders. On the other hand, detecting HCV viremic cases in patients regularly undergoing dialysis for more than four years may indicate a possible role of the hemodialysis process itself. Overall, systematic screening for HCV markers before the first dialysis should help narrow down the likely sources of HCV infection in these patients. In the absence of such data, this study was not able to investigate the relationship between HCV and renal injury, which could be considered a limitation of the study. The second limitation was the cross-sectional design of the study which did not permit the evaluation of the possible impacts of HCV infection on the survival rates of hemodialysis patients.
Conclusion
This is the first report on the prevalence and molecular evaluation of HCV infection among patients on regular hemodialysis in Bushehr, Iran. Moreover, the participation of all hemodialysis patients resident in this region has increased the generalizability of our results. This study reports an HCV prevalence of 5.4% for anti-HCV antibodies and 0.7% for HCV viremia with genotype 3a among hemodialysis patients in Bushehr. Considering the high seroprevalence of HCV in patients undergoing hemodialysis compared to the non-hemodialysis population, regular screening of hemodialysis patients for HCV infection and prompt treatment of those diagnosed as HCV-infected are recommended to reduce the risk of viral transmission in dialysis settings.
Disclosure
The authors declared no conflicts of interest. This study was funded by the Deputy Research and Affairs of Bushehr University of Medical Sciences, Bushehr, Iran, as per grant number 3226.
Acknowledgments
The authors would like to acknowledge the Deputy Research and Affairs of the Bushehr University of Medical Sciences for providing the grant to conduct this study.
references
- 1. Caragea DC, Mihailovici AR, Streba CT, Schenker M, Ungureanu B, Caragea IN, et al. Hepatitis C infection in hemodialysis patients. Curr Health Sci J 2018 Apr-Jun;44(2):107-112.
- 2. Timofte D, Dragos D, Balcangiu-Stroescu A-E, Tanasescu MD, Gabriela Balan D, Avino A, et al. Infection with hepatitis C virus in hemodialysis patients: an overview of the diagnosis and prevention rules within a hemodialysis center (review). Exp Ther Med 2020 Jul;20(1):109-116.
- 3. Nguyen DB, Bixler D, Patel PR. Transmission of hepatitis C virus in the dialysis setting and strategies for its prevention. Semin Dial 2019 Mar;32(2):127-134.
- 4. Adane T, Getawa S. The prevalence and associated factors of hepatitis B and C virus in hemodialysis patients in Africa: a systematic review and meta-analysis. PLoS One 2021 Jun;16(6):e0251570.
- 5. Rahnavardi M, Hosseini Moghaddam SM, Alavian SM. Hepatitis C in hemodialysis patients: current global magnitude, natural history, diagnostic difficulties, and preventive measures. Am J Nephrol 2008;28(4):628-640.
- 6. Mohseni R, Emami Zeydi A, Ilali E, Adib-Hajbaghery M, Makhlough A. The effect of intradialytic aerobic exercise on dialysis efficacy in hemodialysis patients: a randomized controlled trial. Oman Med J 2013 Sep;28(5):345-349.
- 7. Kargar Kheirabad A, Bahri F, Kargar M, Ghasemzadeh I. Hepatitis C and G virus infection prevalence among hemodialysis patients and associated risk factors in the Hormozgan province of Southern Iran. Hepat Mon 2016 Aug;16(10):e40375.
- 8. Farshadpour F, Taherkhani R, Bakhtiari F. Prevalence and predominant genotype of hepatitis C virus infection and associated risk factors among pregnant women in Iran. Biomed Res Int 2021 Sep;2021:9294276.
- 9. Farshadpour F, Taherkhani R, Ravanbod MR, Eghbali SS. Prevalence and genotype distribution of hepatitis C virus infection among patients with type 2 diabetes mellitus. Med Princ Pract 2018;27(4):308-316.
- 10. Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, et al. Prevalence and trends of transfusion-transmissible viral infections among blood donors in south of Iran: an eleven-year retrospective study. PLoS One 2016 Jun;11(6):e0157615.
- 11. Khodabandehloo M, Roshani D, Sayehmiri K. Prevalence and trend of hepatitis C virus infection among blood donors in Iran: a systematic review and meta-analysis. J Res Med Sci 2013 Aug;18(8):674-682.
- 12. Mirminachi B, Mohammadi Z, Merat S, Neishabouri A, Sharifi AH, Alavian SH, et al. Update on the prevalence of hepatitis C virus infection among Iranian general population: a systematic review and meta-analysis. Hepat Mon 2017;17(2):8.
- 13. Yusoff SM, Bahar R, Hassan MN, Noor NH, Ramli M, Shafii NF. Prevalence of red blood cell alloimmunization among transfused chronic kidney disease patients in hospital Universiti Sains Malaysia. Oman Med J 2020 Sep;35(5):e177.
- 14. Taherkhani R, Farshadpour F. Epidemiology of hepatitis C virus in Iran. World J Gastroenterol 2015 Oct;21(38):10790-10810.
- 15. Ghorbani NR, Djalalinia S, Modirian M, Abdar ZE, Mansourian M, Gorabi AM, et al. Prevalence of hepatitis C infection in Iranian hemodialysis patients: an updated systematic review and meta-analysis. J Res Med Sci 2017 Nov;22:123.
- 16. Ashkani-Esfahani S, Alavian SM, Salehi-Marzijarani M. Prevalence of hepatitis C virus infection among hemodialysis patients in the Middle-East: a systematic review and meta-analysis. World J Gastroenterol 2017 Jan;23(1):151-166.
- 17. Khattab OS. Prevalence and risk factors for hepatitis C virus infection in hemodialysis patients in an Iraqi renal transplant center. Saudi J Kidney Dis Transpl 2008 Jan;19(1):110-115.
- 18. Olut AI, Ozsakarya F, Dilek M. Seroprevalence of hepatitis C virus infection and evaluation of serum aminotransferase levels among haemodialysis patients in Izmir, Turkey. J Int Med Res 2005 Nov-Dec;33(6):641-646.
- 19. Akhtar S, Nasir JA, Usman M, Sarwar A, Majeed R, Billah B. The prevalence of hepatitis C virus in hemodialysis patients in Pakistan: a systematic review and meta-analysis. PLoS One 2020 May;15(5):e0232931.
- 20. Kashem A, Nusairat I, Mohamad M, Ramzy M, Nemma J, Karim MR, et al. Hepatitis C virus among hemodialysis patients in Najran: prevalence is more among multi-center visitors. Saudi J Kidney Dis Transpl 2003 Apr-Jun;14(2):206-211.
- 21. Amin J, Gidding H, Gilbert G, Backhouse J, Kaldor J, Dore G, et al. Hepatitis C prevalence–a nationwide serosurvey. Commun Dis Intell Q Rep 2004;28(4):517-521.
- 22. Ramírez R, Fernández J, Guevara JG, Valderrama LA, León Castro A, Arango Álvarez J, et al. Prevalence of anti-HCV antibodies among patients on dialysis in Cali-Colombia. Rev Colomb Gastroenterol 2010;25(1):14-18.
- 23. Jeele MO, Addow RO, Adan FN, Jimale LH. Prevalence and risk factors associated with hepatitis B and hepatitis C infections among patients undergoing hemodialysis: a single-centre study in Somalia. Int J Nephrol 2021 Nov;2021:1555775.
- 24. Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol 2009 Jan;4(1):207-220.
- 25. Fabrizi F. Hepatitis C virus infection and dialysis: 2012 update. ISRN Nephrol 2012 Dec;2013:159760.
- 26. Farshadpour F, Taherkhani R. Prevalence and molecular evaluation of hepatitis C virus infection among multi-transfused thalassemia patients in South of Iran. Oman Med J 2022;37(5):e427.
- 27. Taherkhani R, Farshadpour F. Lurking epidemic of hepatitis C virus infection in Iran: a call to action. World J Hepatol 2017 Aug;9(24):1040-1042.