The outbreak of novel SARS-CoV-2 in the latter part of 2019 rapidly turned into a catastrophe of global proportion and has burdened healthcare systems around the world. SARS-CoV-2 is associated with a wide spectrum of disease patterns, ranging from mild disease to life-threatening conditions.1 The complete pathogenesis and complications of this disease have still not been completely elucidated. Apart from the morbidity and mortality caused by the virus itself, it has been noted that patients affected with this virus have a higher susceptibility to bacterial and fungal co-infections.2 Secondary infections are known to occur in various viral illnesses, and SARS-CoV-2 is also associated with varied bacterial and fungal co-infections.3 A magnitude of research has been conducted on SARS-CoV-2 and its management to curb the infections and manage patients judiciously. Rampant use of drugs like corticosteroids, although helpful in restraining disease progression and improving patient survival, have contributed to a surge in secondary bacterial and fungal infections.3
There is increasing evidence pointing towards these secondary fungal infections in COVID-19 patients, especially those who are or were severely ill, patients with preexisting comorbidities like diabetes mellitus (DM), previous parenchymal lung damage, and immunocompromised states.4 Of these secondary infections, there has been a sudden surge in the cases of mucormycosis in the past few months which has risen to the proportion of an epidemic in itself and is associated with an aggressive disease course with a poor prognosis. Mucormycosis is a rare, life-threatening fungal infection that has an established association with uncontrolled DM and immunosuppression.1 Many emerging articles in the literature have established an association between mucormycosis and COVID-19; however, the research is yet to pinpoint whether this increased coinfection should be attributed to infection per se or management modalities like steroid usage and mechanical ventilation, implemented for its treatment.1–5
A similar escalation of suspected mucormycosis cases was observed and herein, we present a series of 10 cases of mucormycosis diagnosed over one week.
Case report
We compile 10 suspected cases of mucormycosis from the head and neck region received in the department of pathology for evaluation. The patients presented with one or more of the following symptoms: facial/periorbital swelling, diminution of vision, ptosis, ophthalmoplegia, and headache. All the cases were either presently COVID-19 positive or were recovered cases of COVID-19 (diagnosed using reverse transcription polymerase chain reaction). We looked for a detailed clinical history, history of comorbidities, medications received, steroid administration, and oxygen requirement during COVID-19 treatment. Routine blood investigations and levels of inflammatory markers were noted. All cases underwent a computed tomography (CT) scan and three cases underwent additional magnetic resonance imaging (MRI) of the paranasal sinuses and/or brain.
Sample received for histopathological examinations included simple surgical debridement, maxillectomy, and orbital exenteration specimens. A thorough sampling of the specimens was done and was examined with hematoxylin and eosin, Grocott-Gomori’s methenamine-silver stain, and periodic acid-Schiff stain.
We received 10 specimens with suspected mucormycosis in a week. Out of these, five cases were of rhino-orbital mucormycosis and five cases involved the paranasal sinuses. The age of the patients ranged from 25 to 70 years and included nine males and one female. Among the 10 cases, six were COVID-19 positive on admission and four presented with symptoms of mucormycosis after recovery from COVID-19. Six were known diabetics and four did not have DM. Five patients received steroids for the management of their COVID-19 infection and four patients required oxygen support. C-reactive protein levels were raised in all patients, with five patients showing a marked increase in C-reactive protein [Table 1].
Table 1: Details of the patients of mucormycosis with their clinicopathologic parameters.
|
1.
|
25
|
Male
|
Previously positive
|
No
|
No
|
No
|
20.00
|
NA
|
NA
|
NA
|
|
2.
|
51
|
Male
|
Positive
|
DM
|
Not known
|
Not known
|
182.40
|
32.0
|
1234.0
|
> 20
|
|
3.
|
60
|
Male
|
Positive
|
DM
|
Not known
|
Not known
|
185.70
|
NA
|
NA
|
NA
|
|
4.
|
31
|
Male
|
Previously positive
|
DM
|
Yes
|
Yes
|
32.00
|
NA
|
NA
|
NA
|
|
5.
|
38
|
Male
|
Positive
|
No
|
Yes
|
Yes
|
135.90
|
115.90
|
560.60
|
1.060
|
|
6.
|
60
|
Female
|
Positive
|
No
|
Not known
|
No
|
198.30
|
495.40
|
1472.0
|
2.17
|
|
7.
|
42
|
Male
|
Previously positive
|
DM
|
Yes
|
No
|
11.43
|
NA
|
NA
|
NA
|
|
8.
|
70
|
Male
|
Positive
|
No
|
Not known
|
Not known
|
163.70
|
3.10
|
742.0
|
2.63
|
|
9.
|
40
|
Male
|
Positive
|
DM, IHD, and thyroid disorder
|
Yes
|
Yes
|
14.20
|
18.60
|
641.60
|
2.97
|
CRP: C-reactive protein; IL: interleukin; DM: diabetes mellitus; IHD: ischaemic heart disease; NA: not available.
Histopathological examination of all the cases revealed broad, non-septate fungal hyphae with right-angled branching in extensive areas of infarction along with angioinvasion, which is the hallmark finding of mucormycosis. Four cases also showed evidence of intraneural invasion, while five cases demonstrated perineural invasion by Mucor. The fungal load was high in the areas of necrosis. Fungal profiles were well-appreciated with periodic acid-Schiff and Grocott-Gomori's methenamine-silver stains. Necrotizing granulomatous inflammation with the formation of epithelioid cell granulomas, foreign-body type of giant cell histiocytes, and lymphocytes surrounding the fungal balls were noted in seven cases. Few cases demonstrated bony invasion, with the presence of fungal hyphae in the inter-trabecular spaces. Infiltration of the muscle by the fungus was also identified in two cases [Figures 1 and 2].
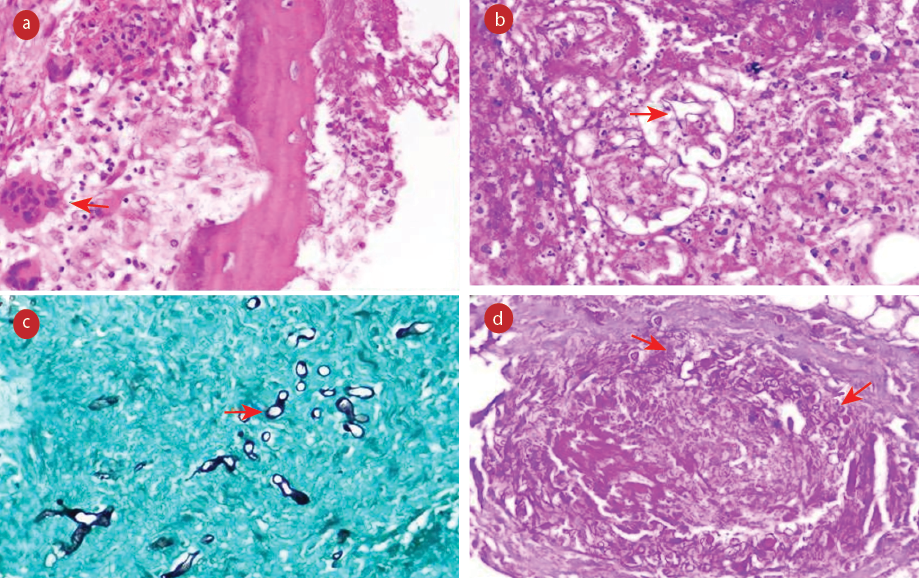 Figure 1: (a) Section showing epithelioid cell granuloma and foreign body giant cell reaction (red arrow) along with fungal hyphae (hematoxylin and eosin (H&E)). (b) Areas of necrosis along with broad fungal hyphae (red arrow) (H&E). (c) Necrotic soft tissue showing scattered fungal hyphae of Mucor (red arrow) Grocott-Gomori’s methenamine-silver. (d) Angioinvasion by fungal hyphae highlighted by special stain (red arrows) periodic acid Schiff, magnification = 400 ×.
Figure 1: (a) Section showing epithelioid cell granuloma and foreign body giant cell reaction (red arrow) along with fungal hyphae (hematoxylin and eosin (H&E)). (b) Areas of necrosis along with broad fungal hyphae (red arrow) (H&E). (c) Necrotic soft tissue showing scattered fungal hyphae of Mucor (red arrow) Grocott-Gomori’s methenamine-silver. (d) Angioinvasion by fungal hyphae highlighted by special stain (red arrows) periodic acid Schiff, magnification = 400 ×.
 Figure 2: (a) Section showing angioinvasion by broad, aseptate fungal hyphae of Mucor species (red arrow) (hematoxylin and eosin (H&E)). (b) Infiltration of muscle by the fungal hyphae (red arrow) ematoxylin and eosin (H&E). (c) A segment of nerve showing intraneural invasion by broad, aseptate fungal hyphae (red arrows) consistent with Mucor species (H&E, oil immersion). (d) Bone invasion with the presence of fungal hyphae in inter-trabecular space (H&E), magnification = 400 ×.
Figure 2: (a) Section showing angioinvasion by broad, aseptate fungal hyphae of Mucor species (red arrow) (hematoxylin and eosin (H&E)). (b) Infiltration of muscle by the fungal hyphae (red arrow) ematoxylin and eosin (H&E). (c) A segment of nerve showing intraneural invasion by broad, aseptate fungal hyphae (red arrows) consistent with Mucor species (H&E, oil immersion). (d) Bone invasion with the presence of fungal hyphae in inter-trabecular space (H&E), magnification = 400 ×.
Discussion
Mucormycosis is a fungal infection caused by saprophytic fungi Mucoraceae, found in the decaying matter in soil, air, and manure.5 Mucormycosis has an incidence of 0.005 to 1.7 per million population with a global fatality rate of around 46%.2 The most common presentation of mucormycosis is rhino-orbital-cerebral infection (44–49%).6 The entry of fungi is via inhalation into the nasal cavity and paranasal sinuses that can further invade into the orbit and brain via blood vessels and nerves. Other forms include cutaneous (10–19%), pulmonary (10–11%), disseminated (6–11%), and gastro-intestinal (2–11%) mucormycosis.6 Common risk factors associated with the development of mucormycosis include uncontrolled DM, diabetic ketoacidosis, HIV/AIDS, iron overload, and immunosuppressive therapies.5
The usual presenting symptoms are fever, facial pain, headache, periorbital swelling, ophthalmoplegia, and ptosis.6
Diagnosis of mucormycosis requires a detailed history, physical examination, and radiological imaging. A CT scan and MRI are essential diagnostic tools to identify the involvement of sinuses, orbit, and brain invasion. Histopathological examination and microbiological cultures are the gold standard for confirmation of Mucor.6 Histopathologically, these are seen as nonseptate, ribbon-like hyphae of variable width (6 to 50 µm) with right-angle branching. The hallmark of mucormycosis on histopathology is the extensive tissue necrosis and infarction caused by angioinvasion and consequent thrombosis.7
The treatment of choice accepted for mucormycosis is intravenous amphotericin B and surgical debridement.5 Some other approved anti-fungal drugs include posaconazole and isavuconazole.7 Isavuconazole being an extended-spectrum anti-fungal is used in the treatment of invasive mucormycosis.
Although an increased spurt of bacterial and fungal co-infections associated with COVID-19 have been reported across the globe, Mucor, in particular, has recently emerged as the most vicious of them all, infecting a large number of recovered and active COVID-19 patients in India. Patients are getting infected more in the later phase of COVID-19 infection.2 These infections are more frequently seen in patients with severe symptoms of COVID-19 infection, those requiring admission to the intensive care unit and mechanical ventilation. The mortality rate is also higher in COVID-19 cases with invasive fungal infection.2
Many authors have pointed out that the increased association of mucormycosis with COVID-19 is attributed to various factors like immune suppression, steroid usage, and comorbidities. There is also increasing evidence pointing to the pathogenesis of COVID-19 infection, which causes immune modulation and plays a conducive role in the development of fungal infection.8
Steroids are known immune-suppressive drugs and have beneficial effects in overcoming hyper-inflammation and the subsequent cytokine storm associated with COVID-19.4 Current guidelines for COVID-19 in India recommend intravenous methylprednisolone 0.5–1 mg/kg/day for three days in moderate cases and 1–2 mg/kg/day in severe cases.3 Severely ill patients who are on oxygen supplementation or ventilation are recommended the use of dexamethasone (6 mg per day for a maximum of 10 days).3 However, the risk of developing secondary infections due to the immunosuppressive nature of glucocorticoids has been specifically indicated in the guidelines.
COVID-19 infection has a propensity to cause immune dysregulation leading to a reduced number of both CD4+T and CD8+T lymphocytes and there can be a marked rise in the level of inflammatory cytokines like interleukin-2, interleukin-6, and tumor necrosis factor-alpha.1
Mucormycosis is known to cause angioinvasion and endothelial damage, which constitutes the main mechanism of its pathogenesis leading to extensive infarction. COVID-19 infection also causes thrombotic microangiopathies. The immunosuppressing effects of steroids along with immune dysregulation caused by COVID-19 and the possible micro thrombotic complications of COVID-19 can form a fertile ground for increased mucormycosis infection.6,9
Mucormycosis is associated with poor prognosis with a mortality rate ranging between 33.3% to 80%.6 This makes the early diagnosis of this infection important, as a delay in diagnosis even for a few days might result in worsening of the prognosis. Even after early diagnosis, together with systemic antifungals and aggressive surgical interventions, the prognosis for recovery from mucormycosis is still poor.
Though steroid usage and the presence of comorbidities are associated with an increased rate of mucormycosis in COVID-19 patients, an alarming observation that we found was some of the patients were infected with Mucor despite the absence of these risk factors. It is not inappropriate to state that weakened immunity post-COVID-19 infection is even responsible for the susceptibility of mucormycosis, irrespective of steroid intake, or DM. The compromised immune system may not be able to fight the Mucor infection upon invasion in post-COVID-19 patients.
conclusion
The situation exploded in India, and the acute rise in the number of cases led to an epidemic within the pandemic. The exact cause of the sudden surge in mucormycosis as a post-COVID-19 sequela is yet to be established. However, identifying high-risk patients and looking out for warning signs can help in early recognition and prevention of this deadly infection.
Disclosure
The authors declared no conflicts of interest. Informed consent was taken from all the patients.
references
- 1. Monte Junior ES, Santos ME, Ribeiro IB, Luz GO, Baba ER, Hirsch BS, et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc 2020 Nov;53(6):746-749.
- 2. Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol 2021 Feb;69(2):244-252.
- 3. Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus 2020 Sep;12(9):e10726.
- 4. Ahmadikia K, Hashemi SJ, Khodavaisy S, Getso MI, Alijani N, Badali H, et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021 Aug;64(8):798-808.
- 5. Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep 2021 May;82(May):105957.
- 6. Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? a retrospective, multi-centric analysis. J Maxillofac Oral Surg 2021 Sep;20(3):418-425.
- 7. Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases 2021 Mar;12(3):85-89.
- 8. Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg 2021 Apr;1-4:1-4.
- 9. Vasquez-Bonilla WO, Orozco R, Argueta V, Sierra M, Zambrano LI, Muñoz-Lara F, et al. A review of the main histopathological findings in coronavirus disease 2019. Hum Pathol 2020 Nov;105:74-83.