The World Health Organization defines an adverse drug reaction (ADR) as “any response to a drug that is noxious and unintended and that occurs at a dose normally used in man for prophylaxis, diagnosis, or treatment of diseases for the modification of physiological function.”1,2 Pharmacovigilance is the monitoring of drug safety, encompassing all activities related to the collection, detection, assessment, monitoring, and prevention of ADRs and other drug-related problems.3 The worldwide incidence of ADRs has been recognized as a substantial cause of lengthy hospitalizations, potentially life-threatening healthcare-related problems and increased healthcare expenditures. This includes additional medical consultations, hospitalizations, laboratory tests, and medications required to manage the ADRs or indirectly to lost productivity due to time off work, transportation expenses to healthcare appointments, and high mortality rates.4,5
Over the last two decades, systematic reviews reported ADR prevalences of 6–24%.6–9 The Institute of Medicine in 2000 reported that in the USA, 44 000–98 000 individuals die each year due to medication errors, of which approximately 7000 die due to preventable ADRs.10 Other studies have placed higher estimates of serious ADRs; they reported the occurrence of serious ADRs in almost 7% of hospitalized patients with a fatality rate of 0.32%, while over 350 000 ADRs occur in USA nursing homes each year, ranking ADRs as the fourth leading cause of death — ahead of respiratory disease, diabetes, HIV, and traffic accidents.11–14
Psychotropic (psychiatric) medications, influencing behavior, mood, thoughts, mental status, or perception, even at therapeutic and maintenance doses, have been linked to several ADRs, most likely due to their prolonged use, impaired clearance, the common practice of using combined therapy, and their proclivity to interact with a wide range of other medications.15–17 These side effects are significant determinants of negative impacts on the patient’s physical and mental health, which eventually may lead to non-adherence to treatment, unmet clinical outcomes, and a substantial rise in the overall cost of healthcare that may reach 6% of the healthcare budget.18
Studies have identified a high burden of psychotropics-related ADRs ranging from 0.7–10%, particularly among patients using second-generation antipsychotics and Selective serotonin reuptake inhibitors.19–23 These side effects may vary in severity from mild to severe dermatologic, cardiovascular, hepatic, sexual, neurological, and hematological. Thomas et al,21 reported that psychiatric medications accounted for 48% of the ADRs in hospitalized patients, while Sridhar et al,15 reported a 10% incidence of ADRs in outpatients.
The National Institute for Health and Care Excellence guidelines recommend monitoring ADRs associated with psychotropics. They have set several parameters to be checked at baseline before initiation of psychotropic medications, after a few months of medication initiation, and then periodically.24 ADR monitoring in hospitalized patients is an important process for identifying patients at high risk of developing ADRs so that tailored interventional strategies can be developed to manage, prevent, and minimize the risk of developing ADRs and maximize clinical outcomes.25
Many factors, including lack of awareness among healthcare professionals and patients, the preoccupation of practitioners on busy wards with other areas of practice, and the work-environment punishment culture, may contribute to the underreporting of ADRs in the hospital setting. Establishing an effective pharmacovigilance program in psychiatry units can be remarkably beneficial in preventing underreporting and promoting the patient safety concept through preventing avoidable harm.26 Therefore, the ultimate objective of this study was to boost pharmacovigilance activity in our psychiatry referral hospital and highlight the role of the pharmacist in ADRs prevention, detection, and management. We aim to promote patient safety and compliance toward psychotropic medication by detecting the pattern of ADRs among hospitalized patients in our psychiatry hospital, assessing and evaluating the causality, severity, and management of the documented ADRs, and establishing a hospital-based ADR reporting platform.
Methods
This retrospective hospital-based study investigates the psychotropic-related ADRs in a tertiary care psychiatry hospital (Al Masarra Hospital, Oman), with a 245-bed capacity comprising general adult psychiatry, child and adolescent psychiatry, geriatrics psychiatry, and forensic psychiatry. The hospital also treats patients who suffer from substance abuse.
Adult patients (18–60 years) admitted between 1 September 2020 and 30 September 2021, who had received at least one psychotropic agent for at least two months were enrolled. Excluded were patients with a history of substance abuse, pregnant females, and those on clozapine therapy due to unique monitoring requirements. A convenient sampling technique was used for sample size calculation.
Electronic patient medical records were examined demographics (age, gender, race), clinical details (diagnosis, admission details, psychotropic treatment, monitoring parameters, any ADRs, and ADR corrective actions). After obtaining ethical approval, psychiatry-trained clinical pharmacists screened the patient’s records for ADRs. The screening of ADRs included all potential side effects that may be developed from medication administrations and their impact on various body systems. All ADRs were assessed for causality, severity, and preventability.
Before 2020, clinical pharmacists at the hospital manually reported ADRs monthly, resulting in a limited number of reports and pharmacist interventions. From 2018 to 2019, only 14 ADRs were officially documented. However, a noteworthy improvement in the reporting of ADRs associated with psychiatric medications was observed in 2020 and 2021 following the implementation of a pharmacist-led ADRs reporting and intervention program. This initiative primarily focused on enhancing pharmacists’ awareness of proactive ADRs reporting and management through comprehensive training sessions. Gathering data posed a formidable challenge, primarily due to the absence of a dedicated ADR reporting channel within the Al-Shifa system. To surmount this obstacle, data acquisition was meticulously conducted through a comprehensive review encompassing clinical summaries, physician notes, and laboratory investigations in conjunction with pharmacist-led clinical rounds, which occurred during the admission of all study participants.
Antipsychotics (first and second generation), mood stabilizers (lithium and anticonvulsants), and antidepressants (selective serotonin reuptake inhibitors and tricyclic antidepressant) were studied.ADRs were analyzed for causality using Naranjo’s algorithm scale27 and for severity using modified Hartwig and Siegel scales.28 Additional assessments for ADR preventability were performed using modified Schumock and Thornton scales.29
Naranjo’s causality assessment identifies the relationship between ADR and the administration of medications. The total score of the causality questions in the table below indicates whether there is a definite, probable, or possible link between ADR and medications (Supplementary 1). Total scores range from -4 to +13. The reaction is considered definite if the score is ≥ 9, probable if 5–8, possible if 1–4, and doubtful if ≤ 0.
A modified Hartwig and Siegel scale is used to assess the severity, it describes the extent to which the ADRs influence the patient’s everyday life. Supplementary 2 shows questions that determine the level of severity of ADR. Seigel and Schneider categorized ADRs into seven severity levels. Levels one and two are less severe, levels three and four are moderate, and levels five, six, and seven are severe.30
Supplementary 3 provides an assessment of preventability in which any question with a yes answer determines whether the ADR is preventable, probable, or not preventable.
Descriptive statistics were applied to present the study results; normally distributed continuous variables were expressed using the mean±SD, whereas when normality is violated, data are expressed as the median and IQR. Categorical data were expressed using frequencies and percentages. The chi-square test was employed to compare the differences in proportions for the categorical variables, whereas the student’s t-test was used to compare the means for normally distributed data, and the Mann-Whitney U-test was used when the data were not normally distributed. A p-value < 0.05 was considered statistically significant. Data were entered and analyzed using (R) Software “Already Tomorrow” version (4.3.0).
This study was approved by the Ministry of Health, Center of Studies and Research Committee, (MH/DGHS/DPT/72/2022) dated 31/06/2022.
Results
During the study period, among 506 admitted adult patients (18–60 years), 327 suspected psychotropic-related ADRs were identified affecting 217 (42.9%) patients. Among them, 130 (59.9%) were men and 87 (40.1%) were women. Hormonal ADRs were five-fold greater in men compared to women (p < 0.001), whereas neurological ADRs had a higher odds ratio among women (p = 0.011). The remainder of the ADR categories were not statistically affected by gender variation [Table 1].
Table 1: Correlation between gender and ADR classes.
|
Hematological
|
8 (2.4)
|
F
|
0 (0.0)
|
*
|
*
|
0.963
|
|
|
M
|
8 (100)
|
*
|
*
|
|
|
Cardiovascular
|
61 (18.7)
|
F
|
23 (37.7)
|
1.26
|
0.71–2.23
|
0.431
|
|
|
M
|
38 (62.3)
|
0.79
|
0.45–1.41
|
|
|
Endocrinological
|
8 (2.4)
|
F
|
4 (50.0)
|
0.72
|
0.18–2.95
|
0.652
|
|
|
M
|
4 (50.0)
|
1.38
|
0.34–5.62
|
|
|
Dermatological
|
3 (0.9)
|
F
|
2 (66.7)
|
0.36
|
0.03–4.03
|
0.408
|
|
|
M
|
1 (33.3)
|
2.76
|
0.25–30.79
|
|
|
Gastrointestinal
|
9 (2.8)
|
F
|
5 (55.6)
|
0.58
|
0.15–2.18
|
0.416
|
|
|
M
|
4 (44.4)
|
1.74
|
0.46–6.59
|
|
|
Hormonal disturbances
|
49 (15.0)
|
F
|
36 (73.5)
|
0.21
|
0.11–0.41
|
< 0.001
|
|
|
M
|
13 (26.5)
|
4.78
|
2.42–9.43
|
|
|
Neurological
|
155 (47.4)
|
F
|
54 (34.8)
|
1.79
|
1.14–2.79
|
0.011
|
|
|
M
|
101 (65.2)
|
0.56
|
0.36–0.87
|
|
|
Organ dysfunction
|
11 (3.4)
|
F
|
3 (27.3)
|
1.99
|
0.52–7.64
|
0.317
|
|
|
M
|
8 (72.7)
|
0.50
|
0.13–1.93
|
|
|
Others
|
23 (7.0)
|
F
|
11 (47.8)
|
0.78
|
0.33–1.83
|
0.572
|
F: female; M: male; ADR: adverse drug reaction; Others: ex hyponatremia and weight gain. *The value is too low to be detected by the analysis software.
The affected patient’s median age (IQR) was 37 (30–46) years; 36 (29–46) for women and 38 (31–47) for men. Patients aged ≤ 39 years had a higher likelihood of cardiovascular ADRs, while those > 39 were more prone to miscellaneous ADRs [Table 2].
Table 2: Correlation between age and ADR classes.
|
Hematological
|
8 (2.4)
|
> 39
|
3 (37.5)
|
0.85
|
0.20–3.62
|
0.826
|
|
|
≤ 39
|
5 (62.5)
|
1.18
|
0.28–5.01
|
|
|
Cardiovascular
|
61 (18.7)
|
> 39
|
16 (26.2)
|
0.44
|
0.24–0.82
|
0.009
|
|
|
≤ 39
|
45 (73.8)
|
2.28
|
1.23–4.23
|
|
|
Endocrinological
|
8 (2.4)
|
> 39
|
2 (25.0)
|
0.47
|
0.09–2.35
|
0.355
|
|
|
≤ 39
|
6 (75.0)
|
2.15
|
0.43–10.79
|
|
|
Dermatological
|
3 (0.9)
|
> 39
|
1 (33.3)
|
0.71
|
0.06–7.89
|
0.780
|
|
|
≤ 39
|
2 (66.7)
|
1.41
|
0.13–15.71
|
|
|
Gastrointestinal
|
9 (2.8)
|
> 39
|
4 (44.4)
|
1.14
|
0.30–4.33
|
0.845
|
|
|
≤ 39
|
5 (55.6)
|
0.88
|
0.23–3.32
|
|
|
Hormonal disturbances
|
49 (15.0)
|
> 39
|
23 (46.9)
|
1.31
|
0.71–2.41
|
0.382
|
|
|
≤ 39
|
26 (53.1)
|
0.76
|
0.41–1.40
|
|
|
Neurological
|
155 (47.4)
|
> 39
|
69 (44.5)
|
1.29
|
0.83–2.00
|
0.260
|
|
|
≤ 39
|
86 (55.5)
|
0.78
|
0.49–1.21
|
|
|
Organ dysfunction
|
11 (3.4)
|
> 39
|
2 (18.2)
|
0.31
|
0.07–1.44
|
0.134
|
|
|
≤ 39
|
9 (81.8)
|
3.27
|
0.69–15.38
|
|
|
Others
|
23 (7.0)
|
> 39
|
15 (65.2)
|
2.88
|
1.18–6.98
|
0.020
|
F: female; M: male; ADR: adverse drug reaction; Others: ex hyponatremia and weight gain.
Combined therapy was associated with high odds of ADRs, only cardiovascular and neurological ADRs were statistically related to monotherapy [Table 3].
Table 3: Correlation between the number of concurrent medications and ADR classes.
|
Hematological
|
8
|
Mono.
|
5 (62.5)
|
0.81
|
0.19–3.44
|
0.771
|
|
|
Comb.
|
3 (37.5)
|
1.24
|
0.29–5.29
|
|
|
Cardiovascular
|
61
|
Mono.
|
48 (78.7)
|
2.02
|
1.04–3.91
|
0.038
|
|
|
Comb.
|
13(21.3)
|
0.50
|
0.26–0.96
|
|
|
Endocrinological
|
8
|
Mono.
|
4 (50.0)
|
0.48
|
0.12–1.94
|
0.302
|
|
|
Comb.
|
4 (50.0)
|
2.10
|
0.51–8.55
|
|
|
Dermatological
|
3
|
Mono.
|
1 (33.3)
|
0.24
|
0.02–2.67
|
0.246
|
|
|
Comb.
|
2 (66.7)
|
4.17
|
0.37–46.52
|
|
|
Gastrointestinal
|
9
|
Mono.
|
7 (77.8)
|
1.73
|
0.35–8.45
|
0.501
|
|
|
Comb.
|
2 (22.2)
|
0.58
|
0.12–2.84
|
|
|
Hormonal disturbances
|
49
|
Mono.
|
33 (67.3)
|
1.00
|
0.53–1.92
|
0.991
|
|
|
Comb.
|
16 (32.7)
|
1.00
|
0.52–1.90
|
|
|
Neurological
|
155
|
Mono.
|
113 (72.9)
|
1.63
|
1.02–2.61
|
0.040
|
|
|
Comb.
|
42 (27.1)
|
0.61
|
0.38–0.98
|
|
|
Organ dysfunction
|
11
|
Mono.
|
5 (45.5)
|
0.39
|
0.12–1.31
|
0.129
|
|
|
Comb.
|
6 (54.5)
|
2.55
|
0.76–8.57
|
|
|
Others
|
23
|
Mono.
|
4 (17.4)
|
0.09
|
0.03–0.26
|
< 0.001
|
Others: ex hyponatremia and weight gain; mono: monotherapy;comb: combined therapy.
The most commonly prescribed psychotropic medications were included in the study, mainly categorized into antipsychotics, antidepressants, and mood stabilizers. Figures 1 and 2a detail the prescribed medications, and related-ADRs prevalence. Antipsychotics were most commonly prescribed (65.3%) followed by mood stabilizers and antidepressants. ADRs were more prevalent with antipsychotic, followed by antidepressants and mood stabilizers.
The identified 327 ADRs represent 34 unique clinical manifestations [Figure 2b] categorized into nine groups [Table 2]. We used Medscape© as an approved reference for adverse reactions associated with the drugs identified,31 and we found that the majority of the ADRs (267, 81.7%) had previously been reported, while some (60, 18.3%) were not registered in the reference database. Figure 3 details the distribution of ADR prevalence as reported by Medscape©.
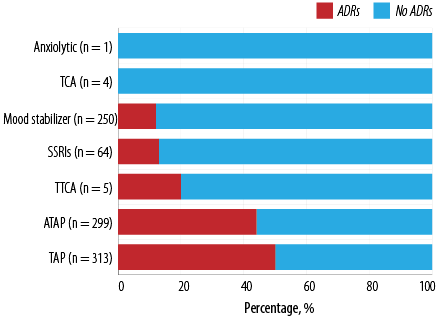 Figure 1: Categories of prescribed psychotropic medications and the percentage of related adverse drug reactions.
Figure 1: Categories of prescribed psychotropic medications and the percentage of related adverse drug reactions.
 Figure 2: (a) Psychotropic medications (prescribed vs. reported adverse drug reactions (ADRs)), and (b) the prevalence of ADRs.
Figure 2: (a) Psychotropic medications (prescribed vs. reported adverse drug reactions (ADRs)), and (b) the prevalence of ADRs.
 Figure 3: Distribution of adverse drug reaction prevalence as reported by Medscape.
Figure 3: Distribution of adverse drug reaction prevalence as reported by Medscape.
Neurological ADRs predominated (155, 47.4%), and most commonly presented as extrapyramidal side effect (EPS) (128, 82.6%) and were closely linked to the use of first-generation (103, 66.5%) and second-generation antipsychotics (45, 29.0%). Fluphenazine (35, 22.5%), risperidone (35, 22.6%), and haloperidol (33, 21.3%) were statistically significant causatives of neurological ADRs, p = 0.001, p = 0.005, and p = 0.001, respectively, the same statistical impact was copied with the EPS [Table 4].
Table 4: The statistical relationship between medications and ADRs.
|
Hematological
|
2 (6.3)
|
0.179
|
2 (2.0)
|
1.000
|
|
|
|
|
|
|
|
|
1 (8.3)
|
0.261
|
|
Thrombocytopenia
|
|
|
|
|
|
|
|
|
|
|
|
|
1 (8.3)
|
0.037
|
|
Neutropenia
|
2 (6.3)
|
0.077
|
2 (2.0)
|
0.641
|
|
|
|
|
|
|
|
|
|
|
|
Cardiovascular
|
10 (31.3)
|
0.090
|
27 (27.3)
|
0.013
|
6 (12.8)
|
0.316
|
2 (8.3)
|
0.275
|
6 (12.2)
|
0.239
|
8 (25.0)
|
0.341
|
|
|
|
QT prolongation
|
1 (3.1)
|
0.404
|
2 (2.0)
|
0.641
|
1 (2.1)
|
0.542
|
|
|
|
|
1 (3.1)
|
0.404
|
|
|
|
Bradycardia
|
|
|
1 (1.0)
|
1.000
|
|
|
1 (4.2)
|
0.205
|
|
|
1 (3.1)
|
0.267
|
|
|
|
Tachycardia
|
2 (6.3)
|
0.293
|
4 (4.0)
|
0.740
|
|
|
1 (4.2)
|
0.573
|
2 (4.1)
|
0.673
|
1 (3.1)
|
1.000
|
|
|
|
Hypotension
|
|
|
6 (6.1)
|
0.072
|
1 (2.1)
|
1.000
|
|
|
2 (4.1)
|
0.650
|
1 (3.1)
|
1.000
|
|
|
|
Hypertension
|
|
|
1 (1.0)
|
0.303
|
|
|
|
|
|
|
|
|
|
|
|
Dyslipidemia
|
7 (21.9)
|
0.015
|
13 (13.1)
|
0.090
|
4 (8.5)
|
1.000
|
|
|
2 (4.1)
|
0.279
|
3 (9.4)
|
1.000
|
|
|
|
Endocrinological
|
4 (12.5)
|
0.004
|
2 (2.0)
|
1.000
|
|
|
|
|
1 (2.0)
|
1.000
|
|
|
|
|
|
Hyperthyroidism
|
1 (3.1)
|
0.186
|
1 (1.0)
|
0.514
|
|
|
|
|
|
|
|
|
|
|
|
Hypothyroidism
|
1 (3.1)
|
0.186
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hyperglycemia
|
2 (6.3)
|
0.049
|
1 (1.0)
|
1.000
|
|
|
|
|
1 (2.0)
|
0.479
|
|
|
|
|
|
Dermatological
|
1 (3.1)
|
0.267
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Itching
|
1 (3.1)
|
0.098
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gastrointestinal
|
3 (9.4)
|
0.048
|
2 (2.0)
|
0.728
|
|
|
|
|
|
|
|
|
|
|
|
Vomiting
|
|
|
1 (1.0)
|
0.303
|
|
|
|
|
|
|
|
|
|
|
|
Conistipation
|
2 (6.3)
|
0.009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Epigastric pain
|
1 (3.1)
|
0.464
|
1 (1.0)
|
0.672
|
|
|
|
|
|
|
|
|
|
|
|
Hormonal disturbance
|
1 (3.1)
|
0.064
|
26 (26.3)
|
0.001
|
5 (10.6)
|
0.508
|
7 (29.2)
|
0.067
|
3 (6.1)
|
0.080
|
3 (9.4)
|
0.443
|
4 (33.3)
|
0.088
|
|
Hyperprolactinemia
|
1 (3.1)
|
0.335
|
17 (17.2)
|
0.002
|
4 (8.5)
|
1.000
|
3 (12.5)
|
0.472
|
3 (6.1)
|
0.593
|
2 (6.3)
|
0.752
|
|
|
|
Amenorrhea
|
|
|
1 (1.0)
|
1.000
|
|
|
2 (8.3)
|
0.015
|
|
|
|
|
|
|
|
Galactorrhea
|
|
|
5 (5.1)
|
0.177
|
1 (2.1)
|
1.000
|
2 (8.3)
|
0.162
|
|
|
1 (3.1)
|
1.000
|
1 (8.3)
|
0.316
|
|
Hirsutism
|
|
|
1 (1.0)
|
1.000
|
|
|
|
|
|
|
|
|
2 (16.7)
|
0.004
|
|
Sexual dysfunction
|
|
|
2 (2.0)
|
0.219
|
|
|
|
|
|
|
|
|
1 (8.3)
|
0.106
|
|
Neurological
|
|
|
35 (35.4)
|
0.005
|
33 (70.2)
|
0.001
|
15 (62.5)
|
0.141
|
35 (71.4)
|
0.001
|
17 (53.1)
|
0.577
|
1 (8.3)
|
0.006
|
|
Tremors/rigidity
|
2 (6.3)
|
0.217
|
2 (2.0)
|
0.728
|
1 (2.1)
|
1.000
|
1 (4.2)
|
0.501
|
1 (2.0)
|
|
|
|
|
|
|
Oversedation
|
2 (6.3)
|
0.293
|
4 (4.0)
|
0.740
|
|
|
|
|
|
|
1 (3.1)
|
1.000
|
1 (8.3)
|
0.342
|
|
Hypersalivation
|
2 (6.3)
|
0.049
|
1 (1.0)
|
1.000
|
|
|
|
|
|
|
|
|
|
|
|
Anticholinergic effects
|
2 (6.3)
|
0.026
|
|
|
|
|
|
|
1 (2.0)
|
0.387
|
|
|
|
|
|
Extrapyramidal Syndrome
|
1 (3.1)
|
0.001
|
28 (28.3)
|
0.009
|
32 (68.1)
|
0.001
|
14 (58.3)
|
0.052
|
33 (67.3)
|
0.001
|
16 (50.0)
|
0.189
|
|
|
|
Organ dysfunction
|
|
|
3 (3.0)
|
1.000
|
|
|
|
|
2 (4.1)
|
0.673
|
1 (3.1)
|
1.000
|
2 (16.7)
|
0.057
|
|
Elevated ALT
|
|
|
3 (3.0)
|
1.000
|
|
|
|
|
2 (4.1)
|
0.650
|
1 (3.1)
|
0.481
|
2 (16.7)
|
0.047
|
|
Others
|
2 (6.3)
|
1.000
|
|
|
3 (6.4)
|
1.000
|
|
|
2 (4.1)
|
0.549
|
|
|
4 (33.3)
|
0.006
|
|
Weight gain
|
2 (6.3)
|
0.049
|
1 (1.0)
|
0.018
|
|
|
|
|
|
|
|
|
1 (8.3)
|
0.140
|
Cardiovascular ADRs ranked second (61, 18.7%) and were more common among second generation antipsychotics (37, 60.7%), with dyslipidemia accounting for most events (29, 47.5%). Although risperidone (27, 44.2%), and olanzapine (10, 16.4%) were noticeable contributors to this category of ADRs, only risperidone was statistically correlated (p = 0.013) [Table 4].
Hormonal disturbances (49, 15.0%) ranked third, manifesting primarily as hyperprolactinemia (30, 61.2%) and galactorrhea (10, 20.4%). The highest proportions of hormonal ADRs were caused by risperidone (26, 53.1%) which demonstrated a high statistically significant relationship with neurological ADRs (p = 0.001), especially hyperprolactinemia (p = 0.002). Other miscellaneous ADRs account for the remaining 18.9% [Table 4].
According to the Naranjo algorithm, 22.9% of ADRs were definite relative to the suspected medication, while the majority (74.3%) were probable [Figure 4].
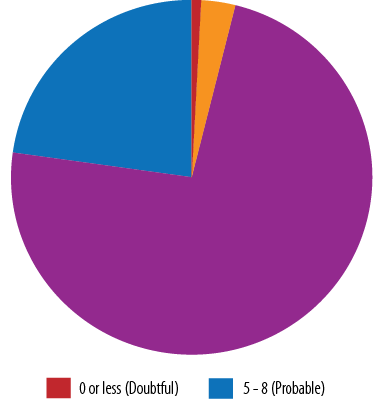 Figure 4: Assessment of causality using the Naranjo algorithm scale.
Figure 4: Assessment of causality using the Naranjo algorithm scale.
According to the Hartwig severity assessment scale, 74.0% were reported as moderate ADRs and 26.0% as mild. No severe reaction was reported [Figure 5].
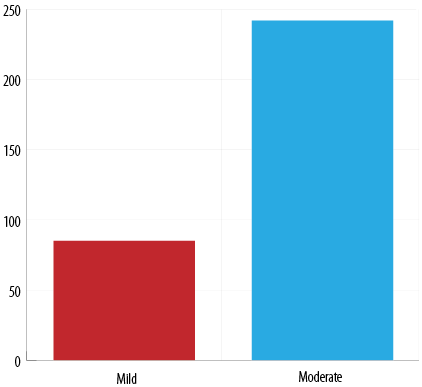 Figure 5: Assessment of severity by using the Hartwig and Seigel scale.
Figure 5: Assessment of severity by using the Hartwig and Seigel scale.
The modified Schumock and Thornton assessment questionnaire revealed that 75.2% of ADRs were unpreventable, 19.3% were probably preventable, and 5.5% were preventable (5.5%) [Figure 6].
 Figure 6: Preventability assessment using the Schumock and Seigel scale.
Figure 6: Preventability assessment using the Schumock and Seigel scale.
This study aimed to report the role of the clinical pharmacist in tracking and managing ADRs, as well as the prevalence of ADRs, which would not have been possible unless we made efforts to promote the culture of tracking and reporting ADRs among pharmacists, as well as other healthcare professionals.
In nearly half of the cases, a new medication was required to manage the emerging ADR; procyclidine and bromocriptine were the most commonly prescribed adjuvants to manage EPS manifestations and hyperprolactinemia, respectively. Other transient conditions were managed using anti-dyslipidemics, hypoglycemics, and antacids. One-third of ADRs required discontinuation of the suspected medication and replacement with an alternative option. Close monitoring without any pharmacological intervention was sufficient in 23.2% of the cases, while dose reduction or dosage form modification was the solution in 7.6% of cases. Examining the patient’s electronic records revealed a high level of healthcare professionals’ commitment (95–100%) to request and follow-up on the laboratory investigation required for therapeutic and ADR monitoring [Figure 7].
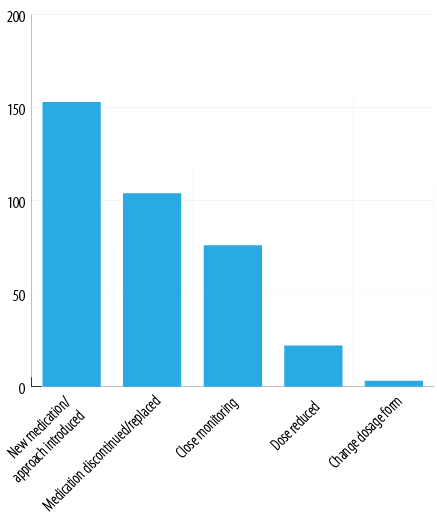 Figure 7: Pharmacist’s interventions.
Figure 7: Pharmacist’s interventions.
Discussion
This is the first large national study to investigate the incidence of psychotropics-related ADRs. A review of 937 medication orders corresponding to 506 admitted patients by psychiatry-trained clinical pharmacists revealed a prevalence of 34.9% ADRs corresponding to 42.9% of patients. The incidence of psychiatric medication-related adverse reactions varied greatly across the available literature. While comparable percentages were reported by some studies,32,33 others reported a range of 5–25% of psychotropic-related ADRs.19,34–37 Extreme percentages were reported by a few studies.38,39 This dramatic disparity in reported proportions can be explained by variations in patient demographics, case severity, healthcare standards, the drugs monitored in each study, and the differences in reporting, monitoring, and follow-up systems. Therefore, relying on standardized work strategies and procedures that enable monitoring, evaluating, and proposing solutions to such events remains the most effective intervention.
Advanced age, polypharmacy, liver and renal impairment, and gender are among the established risk factors for developing ADRs. Several studies have shown that ADRs are more common in women, particularly those brought on by the use of cardiac and psychotropic drugs.40,41 Although there is no definite explanation, the differences between genders in physiological functions such as pharmacokinetic, immunological, and hormonal factors, lower lean body mass, and reduced hepatic clearance are possible explanations for such variance.42,43 This was highlighted by the younger age of the affected women in the study sample compared to the men. In our study, hormonal ADRs were five-fold in men compared to women (p < 0.001), men were more likely to experience non-statistically significantly higher odds of endocrinological, dermatological, and gastrointestinal ADRs. Meanwhile, neurological ADRs were considerably more common in women (p = 0.011).
In our study, the majority (67.3%) of the patients were on psychotropic monotherapy. Combined therapy was associated with non-statistically significant high odds of ADRs; only cardiovascular and neurological ADRs were statistically related to monotherapy probably due to the low number of patients in the combined therapy category.44 Guo et al,45 replicated our findings, reporting an increased risk of psychotropic-related ADRs when using combinations of risperidone, olanzapine, and haloperidol.In the same context, Stingl et al,46 linked using combinations of psychotropics to potential harm in the elderly, which may lead to hospital emergency visits. Contrarily, the study by McCue et al,47 showed that patients being treated with more than one antipsychotic were less susceptible to ADRs and more likely to have improved indicators of patient outcome.
Antipsychotics were the most commonly prescribed psychotropic drugs (65.3%), with both first and second-generation antipsychotics being equally distributed. ADRs appeared in almost 47% of the patients using antipsychotics, and this group was solely responsible for 81.7% of the reported ADRs. Many studies reported a wide range (5–70%) of antipsychotic-related ADRs,48–51 mainly EPS, and hormonal ADRs. Bahta et al,51 linked the high prevalence of ADRs among antipsychotics to medication non-adherence in more than one-third of schizophrenic patients. Therapeutic drug monitoring and non-pharmacological treatments remain preferable options to slow the presumed high prevalence of ADRs related to antipsychotics.52–54
EPS produced by first-generation antipsychotics was the most prominent neurological ADR, frequently a major cause of non-adherence to therapy, resulting in disease relapse and hospitalization. Advanced age, female gender, and high doses of antipsychotics have been identified as the main risk factors for EPS.55,56 Janno et al,57 and other studies linked the high prevalence of EPS in schizophrenic patients to the use of first-generation antipsychotics.58,59 Fluphenazine, haloperidol, and risperidone were statistically significant contributors to the development of EPS in our investigation. Similar findings were replicated with the use of fluphenazine at a dose of 0.2 mg/kg per day, which was linked with higher clinical improvement but also with a significant incidence of EPS.60 Second-generation antipsychotics having the lowest propensity to cause EPS made them an optimal treatment alternative based on the patient’s condition. Effective management of EPS relies mainly on discontinuation or dose reduction of the causative drug or the addition of a central anticholinergic drug, such as procyclidine.
Cardiovascular ADRs were caused mainly by second-generation antipsychotics (risperidone and olanzapine), which coincides with earlier research.61,62 Accurate cardiology assessment of patients receiving these medicines via scheduled electrocardiograms, cardiac enzymes, and lipid profile monitoring remains essential for the early detection and prevention of such problems.
Hyperprolactinemia, menstrual irregularities, and hirsutism were statistically significant reported hormonal ADRs, primarily caused by risperidone, trifluoperazine, and sodium valproate. Causative medication replacement was the main corrective action in the majority of the cases; however, adding bromocriptine remains a common practice to counteract risperidone-related hyperprolactinemia. The frequently reported hormonal disturbances in patients using risperidone63,64 uncover the need for close monitoring and periodic assessment of hormonal levels during treatment.
Other ADRs were statistically linked to the use of certain medications, such as hyperglycemia and constipation with olanzapine, elevated liver enzymes with sodium valproate, and hyponatremia with risperidone, sodium valproate, and lithium. The observed physicians’ high commitment to request and follow-up on patients’ laboratory investigations remains an attitude to be fostered to early recognize and resolve such events. The clinical pharmacist’s role in adopting preplanned therapeutic monitoring of these medications grants maximized outcomes with minor ADRs.65,66
The causality of the reported ADRs indicated that the majority were probable; comparable results were found in a secondary care psychiatric outpatient facility in the UAE15, and a similar pattern was copied in a hospital-based prospective observational study conducted in inpatients and outpatients on antipsychotics.67 Identifying high-risk patient groups, increasing prescribers’ awareness regarding the likelihood of the ADRs related to these drugs, and regular therapeutic monitoring are key tactics to minimize their occurrence.
The vast majority of ADRs in this study were moderate (74.0%), which were managed by the substitution of the used drug or adding another medication to manage the symptoms. The remaining mild cases were managed by reducing the dose and close monitoring of the ADR. Comparable findings were reported by a study in a psychiatry hospital in Kashmir, which reported 83% mild events and 17% moderate.68 Meanwhile, Egbertset al,69 recently reported that 8.3% of serious ADRs related to either labelled or off-label use of psychotropics.
Assessment of preventability showed that most ADRs were unpreventable (75.2%). In a retrospective investigation of long-term resident patients in nursing homes in the USA, 51% of the ADRs were judged to be preventable.12 However, others reported that 57.3% were potentially avoidable.70 This discrepancy is most likely due to variable patient demographics, prescribing patterns, and case severity. Psychotropic medications should be prescribed at the lowest effective dose and not combined unless a single medication is inadequate. In addition, regular and close monitoring of underlying health status, taking into account patient-specific regimen optimization, is essential.
The role of the clinical pharmacist encompasses the identification, monitoring, management, and causality assessment of ADRs in patients experiencing psychiatric illnesses with a great need to implement standardized tools to do so.71 In this study, pharmacists made strenuous efforts to track side effects in the absence of an official documentation tool by directly following up with patients and reviewing their records during the admission period. Similar findings emerged in other countries with established reporting systems and well-trained clinical pharmacists.72–74 The current study contributes valuable insights to the existing body of knowledge regarding the prevalence of ADRs. Furthermore, it underscores the indispensable role of clinical pharmacists in diligently monitoring and effectively addressing ADRs within resource-constrained work environments.
Conclusion
Psychotropics are associated with a wide variety of ADRs, which may contribute to unmet clinical outcomes due to nonadherence to treatment. ADR monitoring in the psychiatry setting by a multidisciplinary team helps to recognize the initial signs of ADRs and hence contributes to better compliance. Hospital-based ADR reporting programs or data capture tools will help in the spontaneous and active assessment and reporting of ADRs by healthcare practitioners.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf 2005;28(10):851-870.
- 2. Ferner RE, McGettigan P. Adverse drug reactions. BMJ 2018 Nov;363:k4051.
- 3. Campbell JE, Gossell-Williams M, Lee MG. A review of pharmacovigilance. West Indian Med J 2014 Dec;63(7):771-774.
- 4. Page RL II, Ruscin JM. The risk of adverse drug events and hospital-related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother 2006 Dec;4(4):297-305.
- 5. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001 Mar-Apr;41(2):192-199.
- 6. Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging 2014 Dec;9:2079-2086.
- 7. World Health Organization. Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. World Health Organization; 2002.
- 8. Angamo MT, Chalmers L, Curtain CM, Bereznicki LR. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf 2016 Sep;39(9):847-857.
- 9. Jennings EL, Murphy KD, Gallagher P, O’Mahony D. In-hospital adverse drug reactions in older adults; prevalence, presentation and associated drugs—a systematic review and meta-analysis. Age Ageing 2020;49(6):948-958.
- 10. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, editors. To err is human: building a safer health system. Washington (DC): National Academies Press (US); 2000.
- 11. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998 Apr;279(15):1200-1205.
- 12. Gurwitz JH, Field TS, Avorn J, McCormick D, Jain S, Eckler M, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000 Aug;109(2):87-94.
- 13. Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 2002 Apr;24(2):46-54.
- 14. Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol 2017 Jun;73(6):759-770.
- 15. Sridhar SB, Al-Thamer SS, Jabbar R. Monitoring of adverse drug reactions in psychiatry outpatient department of a secondary care hospital of Ras Al Khaimah, UAE. J Basic Clin Pharm 2016 Jun;7(3):80-86.
- 16. Roden DM, George Jr AL. The genetic basis of variability in drug responses. Nat Rev Drug Discov 2002 Jan 1;1(1):37-44.
- 17. Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J 2014 Apr 1;22(2):83-94.
- 18. Behera SK, Rath B, Biswal SB, Mohapatra S. Pattern of adverse drug reactions in a tertiary care hospital in Western Odisha. Int J Pharm Sci Res 2018 Jun 1;9(6):2471-2477.
- 19. Barvaliya S, Panchal JR, Desai MK, Parikh M. Pattern of adverse drug reactions into psychiatric patients. Int J Basic Clin Pharmacol 2019 May;8:1059-1066.
- 20. Pahari NI, Tripathi SK, Maity TA, Gupta BK, Bagchi CH, Mondal DK. Evaluation and analysis of adverse drug reactions of second generation antipsychotics in a psychiatry out-patient department. Aggress Behav 2012;6(6):59.
- 21. Thomas M, Boggs AA, DiPaula B, Siddiqi S. Adverse drug reactions in hospitalized psychiatric patients. Ann Pharmacother 2010 May;44(5):819-825.
- 22. Sarumathy S, Menaka K, Samuel GG, Ravichandiran V. A study on drug use pattern and adverse drug reactions of anti-psychiatric medications in a psychiatry specialized hospital. International Journal of Pharmacy and Pharmaceutical Sciences 2014;6(6):332-334.
- 23. Lahon KI, Shetty HM, Paramel AM, Sharma GY. Adverse drug reaction monitoring of antipsychotics, antidepressants and mood stabilizers in the psychiatric outpatient unit of a teaching hospital–a retrospective study. Int J Pharma Bio Sci 2012;3(1):470-478.
- 24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. 2014 [cited 2022 December 25]. Available from: https://www.nice.org.uk/guidance/cg178.
- 25. Chawla S, Kalra BS, Dharmshaktu P, Sahni P. Adverse drug reaction monitoring in a tertiary care teaching hospital. J Pharmacol Pharmacother 2011 Jul;2(3):196-198.
- 26. Sundaran S, Udayan A, Hareendranath K, Eliyas B, Ganesan B, Hassan A, et al. Study on the classification, causality, preventability and severity of adverse drug reaction using spontaneous reporting system in hospitalized patients. Pharmacy (Basel) 2018 Sep;6(4):108.
- 27. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981 Aug;30(2):239-245.
- 28. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992 Sep;49(9):2229-2232.
- 29. Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992 Jun;27(6):538.
- 30. Jones JK. Adverse drug reactions in the community health setting: approaches to recognizing, counseling, and reporting. Fam Community Health 1982 Aug 1;5(2):58-67.
- 31. Medscape. Adverse drug events reporting. 2024 [cited 2023 May 8]. Available from: https://www.medscape.com/resource/adverse-drug-events-reporting.
- 32. Alshehri GH, Keers RN, Ashcroft DM. Frequency and nature of medication errors and adverse drug events in mental health hospitals: a systematic review. Drug Saf 2017 Oct;40(10):871-886.
- 33. Du Z, Jiang Y, Shen Y, Zhou Q, Wang S, Zhu H, et al. Reevaluation of adverse drug reactions of psychiatric drugs under the chinese drug volume-based procurement policy. BMC Health Serv Res 2022 Mar;22(1):424.
- 34. Solanke B, Mahatme MS, Dakhale G, Hiware S, Shrivastava M, Waradkar P. Adverse drug reaction profile at psychiatry out-patient department of a tertiary referral centre in Central India. International Journal of Basic & Clinical Pharmacology 2013;2(3):May-June 2013.
- 35. Al Zaabi MS, Sridhar SB, Tadross TM. Assessment of incidence, causality, severity, and preventability of suspected adverse drug reactions to antidepressant medications in a psychiatry outpatient setting of a secondary care hospital. J Pharm Bioallied Sci 2020;12(2):131-138.
- 36. Heck J, Noltemeyer N, Schulze Westhoff M, Deest-Gaubatz S, Schröder S, Krichevsky B, et al. Adverse drug reactions in geriatric psychiatry—retrospective cohort study of a 6-year period. Ir J Med Sci 2023 Dec;192(6):2917-2927.
- 37. Sankhi S, Marasine NR, Sankhi S, Lamichhane R. Adverse drug reaction due to antidepressants among patients with depression in a private psychiatric hospital of Nepal. Biomed Res Int 2020 Nov;2020:6682928.
- 38. Ejeta F, Aferu T, Feyisa D, Kebede O, Siraj J, Hammeso WW, et al. Adverse drug reaction and its predictors among psychiatric patients taking psychotropic medications at the Mizan-Tepi university teaching hospital. Neuropsychiatr Dis Treat 2021 Dec;17:3827-3835.
- 39. Sakiris MA, Sawan M, Hilmer SN, Awadalla R, Gnjidic D. Prevalence of adverse drug events and adverse drug reactions in hospital among older patients with dementia: a systematic review. Br J Clin Pharmacol 2021 Feb;87(2):375-385.
- 40. Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol 1998 Nov;38(11):1003-1009.
- 41. Drici M-D, Clément N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf 2001;24(8):575-585.
- 42. Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet 2002;41(5):329-342.
- 43. Ghazeeri G, Abdullah L, Abbas O. Immunological differences in women compared with men: overview and contributing factors. Am J Reprod Immunol 2011 Sep;66(3):163-169.
- 44. Rodrigues MC, Oliveira Cd. Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: an integrative review. Rev Lat Am Enfermagem 2016 Sep;24:e2800.
- 45. Guo JJ, Wu J, Kelton CM, Jing Y, Fan H, Keck PE, et al. Exposure to potentially dangerous drug-drug interactions involving antipsychotics. Psychiatr Serv 2012 Nov;63(11):1080-1088.
- 46. Stingl JC, Just KS, Schurig M, Böhme M, Steffens M, Schwab M, et al. Prevalence of psychotropic drugs in cases of severe adverse drug reactions leading to unplanned emergency visits in general hospitals. Pharmacopsychiatry 2020 Apr;53(3):133-137.
- 47. McCue RE, Waheed R, Urcuyo L. Polypharmacy in patients with schizophrenia. J Clin Psychiatry 2003 Sep;64(9):984-999.
- 48. Peyrière H, Roux C, Ferard C, Deleau N, Kreft-Jais C, Hillaire-Buys D, et al; French Network of the Pharmacovigilance Centers. Antipsychotics-induced ischaemic colitis and gastrointestinal necrosis: a review of the French pharmacovigilance database. Pharmacoepidemiol Drug Saf 2009 Oct;18(10):948-955.
- 49. Rafaniello C, Pozzi M, Pisano S, Ferrajolo C, Bertella S, Sportiello L, et al. Second generation antipsychotics in ‘real-life’ paediatric patients. Adverse drug reactions and clinical outcomes of drug switch. Expert Opin Drug Saf 2016 Dec;15(sup2):1-8.
- 50. Sekine Y, Rikihisa T, Ogata H, Echizen H, Arakawa Y. Correlations between in vitro affinity of antipsychotics to various central neurotransmitter receptors and clinical incidence of their adverse drug reactions. Eur J Clin Pharmacol 1999 Oct;55(8):583-587.
- 51. Bahta M, Ogbaghebriel A, Russom M, Tesfamariam EH, Berhe T. Impact of adverse reactions to first-generation antipsychotics on treatment adherence in outpatients with schizophrenia: a cross-sectional study. Ann Gen Psychiatry 2021 Apr;20(1):27.
- 52. Spina E, Hiemke C, de Leon J. Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol 2016 Apr 2;12(4):407-422.
- 53. Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, Perez-Iglesias R, Martinez-Garcia O, Perez-Pardal T, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry 2006;67(8):1253-1260.
- 54. Lucca JM, Madhan R, Parthasarathi G, Ram D. Identification and management of adverse effects of antipsychotics in a tertiary care teaching hospital. J Res Pharm Pract 2014;3(2):46-50.
- 55. Hedenmalm K, Güzey C, Dahl ML, Yue QY, Spigset O. Risk factors for extrapyramidal symptoms during treatment with selective serotonin reuptake inhibitors, including cytochrome P-450 enzyme, and serotonin and dopamine transporter and receptor polymorphisms. J Clin Psychopharmacol 2006 Apr;26(2):192-197.
- 56. Salem H, Nagpal C, Pigott T, Teixeira AL. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol 2017;15(5):789-798.
- 57. Janno S, Holi M, Tuisku K, Wahlbeck K. Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am J Psychiatry 2004 Jan;161(1):160-163.
- 58. Kotomin I, Korotkov A, Solnyshkina I, Didur M, Cherednichenko D, Kireev M. Parkinson’s disease-related brain metabolic pattern is expressed in schizophrenia patients during neuroleptic drug-induced parkinsonism. Diagnostics (Basel) 2023;13(1):74.
- 59. Ali T, Sisay M, Tariku M, Mekuria AN, Desalew A. Antipsychotic-induced extrapyramidal side effects: a systematic review and meta-analysis of observational studies. PLoS One 2021 Sep;16(9):e0257129.
- 60. Levinson DF, Simpson GM, Singh H, Yadalam K, Jain A, Stephanos MJ, et al. Fluphenazine dose, clinical response, and extrapyramidal symptoms during acute treatment. Arch Gen Psychiatry 1990 Aug;47(8):761-768.
- 61. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf 2000;23(3):215-228.
- 62. Feinstein RE. Antipsychotic medications. Heart Disease 2002;4:134-190.
- 63. Kearns AE, Goff DC, Hayden DL, Daniels GH. Risperidone-associated hyperprolactinemia. Endocr Pract 2000;6(6):425-429.
- 64. Margari L, Matera E, Petruzzelli MG, Simone M, Lamanna AL, Pastore A, et al. Prolactin variations during risperidone therapy in a sample of drug-naive children and adolescents. Int Clin Psychopharmacol 2015 Mar;30(2):103-108.
- 65. Lan T, Wang H, Li X, Yin H, Shao D, Jiang Y, et al. The effect of clinical pharmacists’ intervention in adverse drug reaction reporting: a retrospective analysis with a 9-year interrupted time series. BMC Health Serv Res 2022 Jul;22(1):925.
- 66. Karuppannan M, Mohamad Rizal NA, Wong KT, Mohd. Ali S, Ting KN, Boardman H. Pharmacists’ experiences on adverse drug reaction: 10 years later. Front Pharmacol 2022 Sep 29;13:932942.
- 67. Subeesh V, Maheswari E, Singh H, Beulah E. Adverse drug reactions due to atypical antipsychotics in the absence of other centrally acting drugs among patients with mental illness. Arch Pharm Pract 2019;10(2):105-109.
- 68. Afkat A, Arshad H, Samina F, Shagufta P, Vineeta S, Zubair A. Prevalence and severity of adverse drug reactions (ADRs) in patients subjected to different anti-psychotic drugs in an out-patient department of a psychiatry hospital in Kashmir; a prospective observational study. International Journal of Pharmacology and Clinical Sciences 2016;5(1).
- 69. Egberts KM, Gerlach M, Correll CU, Plener PL, Malzahn U, Heuschmann P, et al. Serious adverse drug reactions in children and adolescents treated on-and off-label with antidepressants and antipsychotics in clinical practice. Pharmacopsychiatry 2022;55(05):255-265.
- 70. Ahern F, Sahm LJ, Lynch D, McCarthy S. Determining the frequency and preventability of adverse drug reaction-related admissions to an Irish University Hospital: a cross-sectional study. Emerg Med J 2014 Jan;31(1):24-29.
- 71. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med 2006;166(9):955-964.
- 72. Singh H, Yacob M, Sabu L. Adverse drug reactions monitoring of psychotropic drugs: a tertiary care centre study. OJPAS 2017;8(2):136-140.
- 73. Jayanthi C, Divyashree M, Sushma M. Adverse drug reactions in psychiatry outpatients: clinical spectrum, causality and avoidability. J Chem Pharm Res 2013;5(8):128-135.
- 74. Piparva KG, Buch J, Chandrani KV. Analysis of adverse drug reactions of atypical antipsychotic drugs in psychiatry OPD. Indian J Psychol Med 2011;33(2):153-157.