| |
Abstract
Objective: While SLE is found worldwide, there is diversity in clinical presentation of the disease according to geographical variations. The aim of this study is to describe geographical distributions of childhood onset SLE within Oman to identify geographical clustering and to compare the demographic, clinical, and immunological characteristics of this cluster against the rest of Oman.
Methods: We retrospectively reviewed the hospital charts of 104 consecutive children with childhood onset SLE who were seen in pediatric rheumatology centers in the Sultanate of Oman over a 15-year period between 1995 and 2010.
Results: Geographical clustering of childhood onset SLE was identified in Sharqiya region, which constituted 41% (n=43) of all cases in Oman. This cohort of patients had characteristic disease features which consisted of significantly more boys affected with SLE compared to the rest of the country (42% versus 15%; p=0.002). These children also tended to be younger (10.3 versus 16.5 years; p=0.001), diagnosed at an earlier age (6.4 versus 9.4 years; p<0.001) with a stronger family history of SLE (58% versus 33%; p=0.010). These children also had increased incidence of mucocutanous changes (81% versus 62%; p=0.036) and decreased hematological abnormalities (30% versus 51%; p=0.036).
Conclusion: We identified geographical clustering of childhood onset SLE to Sharqiya region in Oman which is associated with unique demographical and clinical features. Whether increased prevalence of disease in this region is due to geographical, environmental, ethnic or genetic factors is yet to be determined. However, it is likely to be interplay of known and other unrecognized factors.
Keywords: SLE; Oman; Lupus; Demography.
Introduction
There is a worldwide variation in the incidence of childhood manifestation of systemic lupus erythematosus (SLE), which may be related to environmental, genetic and geographical factors. There is now enough evidence to support ethnic and geographical variations in the manifestations of SLE including disease frequency, serological changes, organ involvement and overall prognosis.1-3
Oman is an Arab country situated in the Middle East. It has a unique geographical variation. The coast is formed by the Arabian Sea on the south and east and the Gulf of Oman on the northeast. Oman is divided administratively into four governorates (Muscat, Dhofar, Musandam and Buraimi) and five regions (Batina, Dhahira, Dakhiliya, Sharqiya and Wusta). The aim of this study is to describe geographical distribution of childhood onset SLE within Oman in order to identify if geographical clustering of the disease exists and to compare the demographic, clinical, and immunological characteristics of this geographical cluster against the rest of Oman.
Methods
We retrospectively reviewed the hospital charts of 104 consecutive children with childhood onset SLE who were seen in pediatric rheumatology centers in the Sultanate of Oman over a 15-year period between 1995 and 2010. There are only two pediatric rheumatology referral centers in the country, Royal Hospital and Sultan Qaboos University Hospital both located in the capital region of Muscat. This study includes all childhood onset SLE patients seen in both centers, which represents all SLE patients in the country within the defined period. All the subjects included in the study were Omani children below 13 years of age that is the cut of limit for the pediatric age group in most Arabian countries. All children fulfilled at least four of the American College of Rheumatology (ACR) criteria,4 and have been diagnosed with SLE at least six months prior to inclusion in the study. Evaluation of clinical criteria and immunological parameters were made at the time of diagnosis.
A data collection format was designed which included the following parameters: name including tribal surname, regional origin, wilaayat (city), age, gender, age at diagnosis, disease duration, family history of SLE and degree of consanguinity, as well as clinical, laboratory and immunological data. The patients’ parents were called to obtain verbal consent for the research project and to clarify and obtain missing data from chart review including regional origin and family history of SLE with the degree of consanguinity.
Clinical manifestations that were studied were the major clinical manifestations of SLE including mucocutanous, articular, cardiorespiratory, gastrointestinal, hematological and central nervous systems involvement. The immunological parameters recorded include antinuclear antibodies (ANA), which is determined by immunofluorescence using Hep-2 cells as substrate. Anti-double stranded DNA (anti-dsDNA) was measured qualitatively using enzyme linked immunosorbent assay technique. Extractable nuclear antigens including anti-RNP, Sm, SS-A, SS-B, and anticardiolipin were assessed by a standardized ELISA technique. Disease activity was measured using SLEDAI. The SLEDAI has been shown to be a valid and reliable disease activity measure in multiple patients groups, and has also has been shown to be sensitive to changes in disease activity in children.
Descriptive statistics were used to describe the data. For categorical variables, frequencies and percentages were reported. Differences between groups (Sharqiya versus the rest of the Sultanate) were analyzed using Pearson’s χ2 tests (or Fisher’s exact tests for cells less than 5). For continuous variables, means and standard deviations were presented and analyses were conducted using Student’s t-test. An a priori two-tailed level of significance was traditionally set at the 0.05 level. Statistical analyses were conducted using STATA version 11.1 (STATA Corporation, College Station, TX).
Results
Among the 104 children with SLE studied, a total of 43 patients (41%) were from the Sharqiya region. The other three most populous SLE regions were Batina (18%), Muscat (17%), and Dakhiliya (10%). Table 1 illustrates the demographic data of childhood onset SLE in Sharqiya region and compares them to the rest of the Sultanate. The average age of the children was 14.6 ± 6.3 years while the mean age at diagnosis was 8.2 (±3.3) years representing largely young girls (74%). Almost 45 children (43%) had at least one family member diagnosed with SLE according to the ACR criteria. The degree of consanguinity of patients affected with SLE was first degree in 80% (n=36), second degree in 18% (n=8) and third degree in 2% (n=1). There were significantly more boys affected with SLE in the Sharqiya region compared to the rest of the country (42% versus 15%; p=0.002). Children with SLE from the Sharqiya region were also younger (10.3 versus 16.5 years; p=0.001) and diagnosed at an earlier age (6.4 versus 9.4 years; p<0.001) compared to the rest of the sultanate. In addition, children from this region had more cases of familial SLE (58% versus 33%; p=0.010). The degree of consanguinity of cases of familial SLE in Sharqiya region was similar to the rest of the regions in Oman (first degree 84%, second degree 12% and third degree 4%).
The clinical characteristicsof children from Sharqiya region with SLE at diagnosis compared to those from the rest of the Sultanate are summarized in Table 2. The most prominent clinical characteristics of childhood onset SLE in all of Oman were fever (n=57; 55%), mucocutaneous manifestations (n=73; 70%), arthritis (n=63; 61%), nephritis (n=63; 61%), proteinuria (n=64; 62%), hematuria (n=50; 48%), and hematological involvement (n=44; 42%). Although pulmonary involvement occurred in (n=22; 21%), the occurrence of subclinical pulmonary involvement identified by abnormal PFT was higher (79%).5 The unique clinical manifestations of children with SLE from the Sharqiya region were higher incidence of mucocutaneous manifestations when compared to children from the rest of the Sultanate (81% versus 62%; p=0.036). However, they were less likely to have hematological involvement in comparison to children from the rest of the Sultanate (30% versus 51%; p=0.036). Specifically, they were less likely to have thrombocytopenia when compared to children from the rest of the Sultanate (2.3% versus 16%; p=0.026). The difference in the other clinical and laboratory characteristics should be interpreted with caution due to low power.
With regards to serological parameters of the study cohort (table 3), almost all the children were ANA (n=96; 92%) and anti-dsDNA (n=84; 81%) positive. Children with SLE from Sharqiya region tended to have low incidence of Ro/SSA (7% versus 18%; power=26%), La/SSB (7% versus 15%; power=13%), Anti-RNP (7% versus 18%; power=26%), and Anti-Sm (9% versus 20%; power=20%). However, these results should be interpreted with caution due to low power as indicated. With regards to biochemical parameters of the study cohort (table 4), almost all children with SLE had low C3 (86%) and C4 (88%) while 69% had high ESR. Children from the Sharqiya region also tended to have high incidence of low C4 (91% versus 82%; power=18%), high CRP (47% versus 36%; power=16%) and ESR (72% versus 67%; power=5%). However, these results should also be interpreted with caution due to low power as indicated above. Despite apparent significant differences with regards to hematological and mucocutaneous manifestations, disease activity index, as measured by SLEDAI, was not significantly different between the two cohorts (14 versus 14; p=0.950).
Table 1: Demographic characteristics of the SLE children stratified by geography (Sharqiya region versus the rest of Oman) (N=104).
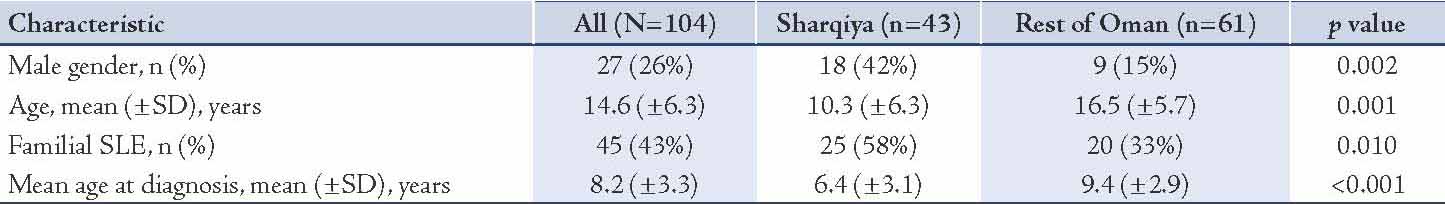
Table 2: Clinical characteristics of the SLE children stratified by geography (Sharqiya region versus the rest of Oman) (N=104).
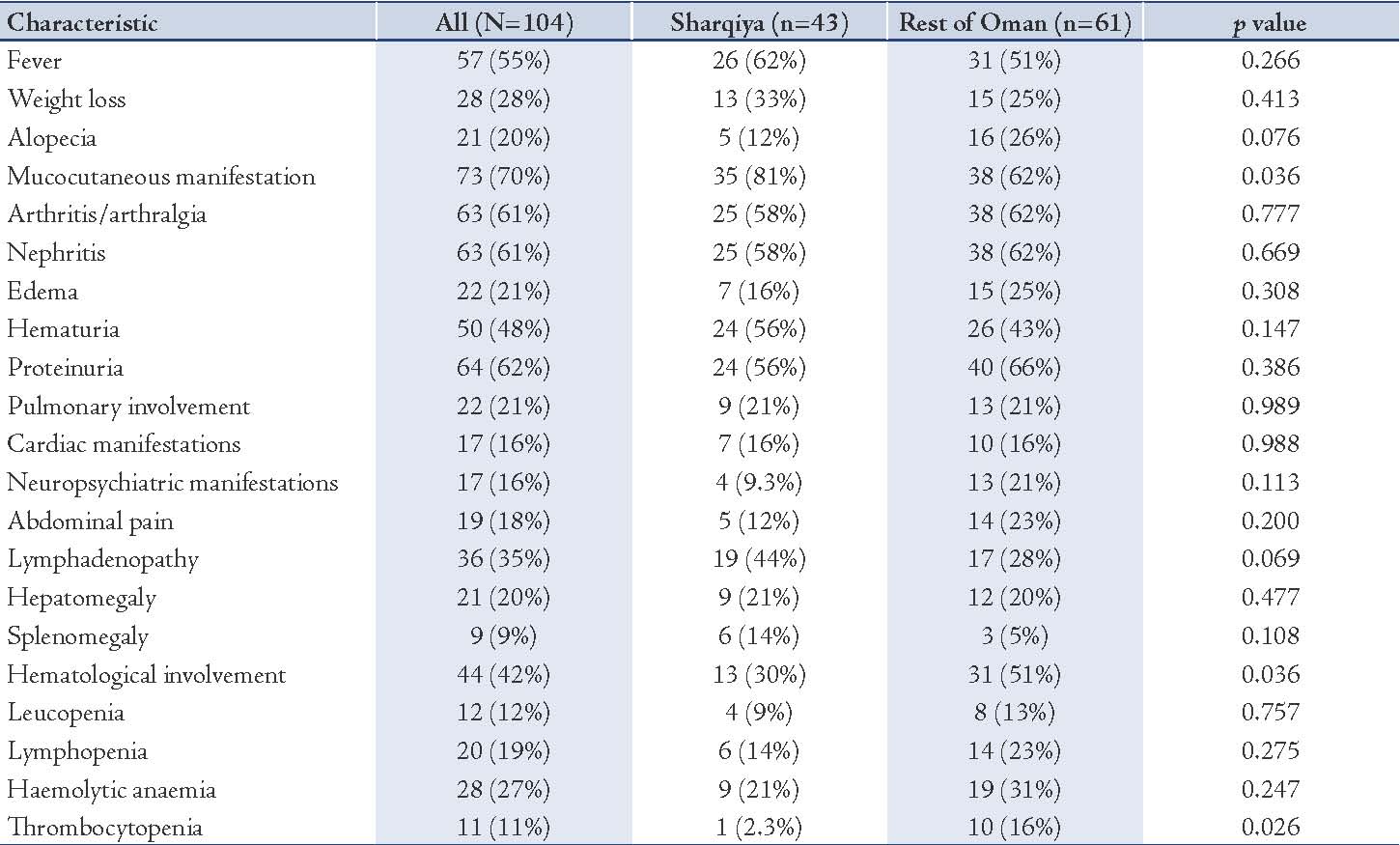
Table 3: Serological characteristics of the SLE children stratified by geography (Sharqiya region versus the rest of Oman) (N=104).
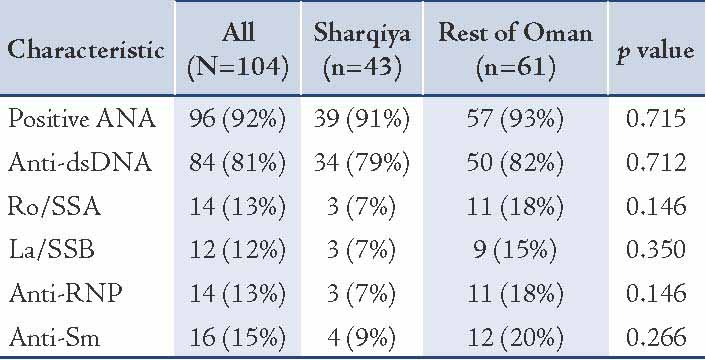
Table 4: Other biochemical parameters of the SLE children stratified by geography (Sharqiya region versus the rest of Oman) (N=104).
Discussion
Preliminary data from Omani census (2010) estimate the population of Oman to be 1.95 million (excluding expatriates).6 With regards to population density in the various regions of the sultanate, Batina region is the most populous region (28.3%) followed by Muscat region (27.3%), Sharqiya region (13%), Dakhiliya region (11.7%), Dhofar region (9.3%), Dhahirah region (5.5%), Wustah region (1.2%) and Musandam region (1.2%).6 Oman has a fairly young population, 42.7% of the population are children below 14 years of age. On a population-adjusted basis (833,000 children), the prevalence of childhood onset SLE per 100,000 Omani children from each governate was: Dakhiliyah (3.17/100,000), Dhahirah (2.71/100,000), Batinah (2.49/100,000), Wusta (3.05/100.000), Sharqiya (12.3/100.000), Dhofar (3.61/100.000), and Muscat (2.45/100.000). Based on population densities, we would expect the number of cases of childhood onset SLE to be more prevalent in Batinah and Muscat region, however, it is surprising that the cases of childhood onset SLE are more prevalent in Sharqiya region (41%), which constitutes only 13% of population of Oman. Whether increased prevalence of disease is due to geographical, environmental, ethnic or genetic factors is yet to be determined.
It is interesting to note that 77% of all cases of childhood onset SLE in Oman come from the regions of Batinah, Muscat and Sharqiya. These regions have more or less the same geographical variations, sharing the Gulf of Oman, and have the same environmental exposure with similar distribution of ultraviolet light. However, these regions also share the same problem, which is pollution of the coastal areas by oil tanker traffic through the Strait of Hormuz and Gulf of Oman.7 It is unknown whether this might contribute to disease causation. With regards to ethnic diversity in Oman, the indigenous population is predominantly Arab except on the Batinah coast, where there is significant Baluchi, Iranian, and African representation, and in Muscat region, Matrah, where there are Khojas and other Indians, Baluchis, and Pakistanis.7,8 However, ethnic diversity which is more prevalent in Batinah and Muscat region do not seem to play a role in disease causation in Oman as the majority (if not all) of the Omani’s from Sharqiya region are of Arab descent. With regards to genetic factors, a rare autosomal recessive form of SLE, in which autozygome analysis revealed a null mutation in the DNASE1L3 gene has been identified in some cases of childhood onset SLE in Oman.9 However, this study was limited to certain families with familial SLE in the sultanate. Whether other genetic factors play a role in disease causation is yet to be determined.
The features of childhood onset SLE in Oman has been described previously and differences were identified among neighboring Arab countries.10 However, it is interesting to note that in addition to geographical clustering of childhood onset SLE in Oman, there are demographic and clinical differences in disease presentation that exists. The children with SLE from Sharqiya region tend to be younger boys (10.3 versus 16.5 years; p=0.001) and diagnosed at an earlier age (6.4 versus 9.4 years; p<0.001). Although, it is assumed that when SLE starts at an earlier age, there tends be a greater load for genetics in the disease pathogenesis, however, this does not seem to influence disease activity at presentation or mortality rate when compared to the rest of the regions in the Sultanate. Another unique feature of the children from Sharqiya region is the gender distribution. Typically, SLE tends to be predominantly a female disease in post pubertal age group (F:M 5:1) and the ratio tends to be equal in pre-pubertal age group (F:M 1.2:1),11 however is it quite unusual to have a male predominance as seen in children from Sharqiya region, which has been rarely reported before and remains unexplained.
Consanguineous marriage is quite prevalent in all Arabian countries including Oman. Because of the strictly endogamous nature of the tribal groups in Oman, all marriages would be expected to be consanguineous to some degree in Oman.12 Consanguineous marriage in Sharqiya region appears to be similar in frequency to the rest of the regions in Oman. However, the frequency of familial cases of childhood onset SLE in the Sharqiya region is relatively higher than the rest of Oman (58% versus 33%; p=0.010). In a previous study, the rate of familial SLE was shown to be higher in Oman (36%) compared to Western countries (10-15%).13 However, there were no clinical or immunological differences between familial and non familial cases of childhood onset SLE in Oman.
In addition to demographic differences, there are clinical differences in the presentation of childhood onset SLE in Sharqiya region compared to the rest of Oman. These children have increased incidence of mucocutanous changes (81% versus 62%; p=0.036), but comparable frequency of serious organ system involvement apart from decreased hematological manifestations (30% versus 51%; p=0.036). There is tremendous variability and diversity in the type of mucocutanous involvement in patients with SLE, ranging from the classic butterfly rash, discoid lupus, cutaneous bullae, oral and nasopharyngeal ulcers, cicatrizing and non-cicatrizing alopecia, and skin changes resulting from vasculitis can all be seen. The most frequent mucocutaneous manifestations of SLE in general are malar rash (40%), alopecia (24%), and oral ulcers (19%).14 However, it is interesting to note that the most common type of rash that present in children with SLE in Oman is urticarial rash (80%) that is diffusely distributed in the face, trunk and extremities which is an uncommon cutanous manifestation of SLE.15,16
Other reports have also demonstrated geographical differences that affect the prevalence of SLE among different regions, notably with the disease being more common in urban than rural areas.17,18 Similarly, Patri et al. case mapped 200 SLE patients in the Midland, UK and located 12 SLE patients residing within an area of one square mile concluding that some geographical areas have greater than expected prevalence of SLE.19 Similarly, there seems to be geographical variation in mortality rate in patients with SLE.20 In the United States, the SLE mortality rate shows great regional variation. Census data showed that the clusters with elevated mortality had higher poverty rates and/or greater concentrations of ethnic Hispanics than those with lower mortality. The variation appears to reflect, in part, geographical patterns in socioeconomic status and in the distribution of persons of Hispanic origin. The contributions of poverty and Hispanic ethnicity to geographical variation of SLE mortality in the United States are substantial, but these factors do not fully account for the clustering phenomenon. Lower socioeconomic status may have other implications that affect outcome which includes difficulty in access to health care, quality of care and compliance with care. Therefore, one has to interpret disease expression and outcome with caution in this subgroup, which may not necessarily be related to difference in racial origin.21 Healthcare in the Sultanate of Oman is predominantly provided and financed by the government. Health care is offered free of cost to all national citizens of Oman. This includes free access to all health care services, facilities and treatment. In 2000, the world health organization (WHO) in assessing the health care systems around the world ranked Oman the eighth in the world for providing a comprehensive health care system. For this reason, we do not speculate that socioeconomic factors contribute to the quality of health care that is delivered to patients in Oman as in other countries. One must consider other contributory factors that will account for the geographical variation of disease prevalence, demographics and clinical presentation. Whether these variations are due to environmental factors, cultural habits or genetic differences is not fully understood, but likely to be interplay of these known and other yet unrecognized factors.
This study is not without limitations. Since subjects were not randomized, its retrospective nature could have introduced bias. However, this bias was minimized as the study attempted to include all the children with SLE followed up in both pediatric rheumatology centers in the country. This study is also largely underpowered due to the small sample size in some aspects, which may not be powerful enough to detect clinically meaningful treatment effects. The uncommon nature of SLE renders it logistically unrealistic to recruit large numbers for the study. However, the study can still provide scientifically meaningful information that may be pooled in future meta-analyses. Clusters or high incidence of SLE areas might yield important clues into the pathogenesis of SLE.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or notfor-profit sectors. The authors declare that they have no conflicts of interest or otherwise to report.
References
1. Lau CS, Yin G, Mok MY. Ethnic and geographical differences in systemic lupus erythematosus: an overview. Lupus 2006;15(11):715-719.
2. McCarty DJ, Manzi S, Medsger TA Jr, Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum 1995 Sep;38(9):1260-1270.
3. Johnson SR, Urowitz MB, Ibañez D, Gladman DD. Ethnic variation in disease patterns and health outcomes in systemic lupus erythematosus. J Rheumatol 2006 Oct;33(10):1990-1995.
4. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982 Nov;25(11):1271-1277.
5. Abdulla E, Al-Zakwani I, Baddar S, Abdwani R. Extent of subclinical pulmonary involvement in childhood onset systemic lupus erythematosus in the sultanate of oman. Oman Med J 2012 Jan;27(1):36-39.
6. Preliminary results of 2010 census Oman (2010) http://www.omancensus.net/new/ Accessed 12 April 2012.
7. World mark encyclopedia of nations. http://www.encyclopedia.com/topic/Oman. Accessed 2 Febuary 2012.
8. "Oman." Encyclopædia Britannica. Encyclopædia Britannica Online (2011) http://www.britannica.com/EBchecked/topic/428217/Oman/45162/People/ Accessed 20 April 2012.
9. Al-Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet 2011 Dec;43(12):1186-1188.
10. Abdwani R, Rizvi SG, El-Nour I. Childhood systemic lupus erythematosus in Sultanate of Oman: demographics and clinical analysis. Lupus 2008 Jul;17(7):683-686.
11. Pluchinotta FR, Schiavo B, Vittadello F, Martini G, Perilongo G, Zulian F. Distinctive clinical features of pediatric systemic lupus erythematosus in three different age classes. Lupus 2007;16(8):550-555.
12. Rajab A, Patton MA. A study of consanguinity in the Sultanate of Oman. Ann Hum Biol 2000 May-Jun;27(3):321-326.
13. Abdwani R, Hira M, Al-Nabhani D, Al-Zakwani I. Juvenile systemic lupus erythematosus in the Sultanate of Oman: clinical and immunological comparison between familial and non-familial cases. Lupus 2011 Mar;20(3):315-319.
14. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002 Jul;20(3):373-385, v. v.
15. Spadoni M, Jacob C, Aikawa N, Jesus A, Fomin A, Silva C. Chronic autoimmune urticaria as the first manifestation of juvenile systemic lupus erythematosus 2011; 20: 763-766.
16. Berti S, Moretti S, Lucin C, Amato L, Massi D, Fabbri P. Urticarial vasculitis and subacute cutaneous lupus erythematosus. Lupus 2005;14(6):489-492.
17. Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 2007 Jun;56(6):2092-2094.
18. Hopkinson ND, Muir KR, Oliver MA, Doherty M, Powell RJ. Distribution of cases of systemic lupus erythematosus at time of first symptom in an urban area. Ann Rheum Dis 1995 Nov;54(11):891-895.
19. Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2002 Dec;16(5):847-858.
20. Walsh SJ, DeChello LM. Geographical variation in mortality from systemic lupus erythematosus in the United States. Lupus 2001;10(9):637-646.
21. Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med 1991 Oct;91(4):345-353.
|
|