Diarrheal diseases in children under the age of five are the second most common cause of death, accounting for 525 000 deaths annually.1 Globally, the incidence of bacterial origins of diarrhea has been documented with varying degrees of occurrence. As an illustration, in South Asian nations, the occurrence has been estimated to be approximately 10%, whereas in Jordan, it reaches 24% among children.2,3
In a multicentered study, Shigella was the most common organism found in bloody diarrhea in children under five in Sub-Saharan Africa and South Asian countries.4 In Oman, the reported prevalence of acute bloody diarrheal illness in a single study from A'Dhahira was 9.1% with no mortalities.5 In 2022, a total of 65 391 diarrheal cases were reported in Oman in under five-year-olds, an increase of 35 episodes per 1000 children compared to 2021. No mortalities were reported.6
Acute febrile bloody diarrhea can be caused by multiple pathogens. Shigella is the second most common cause of diarrhea mortality and morbidity, accounting for 60 000 deaths in children under five annually.7 A recent study in Africa and Asia showed the most common organisms for acute diarrhea were rotavirus, Cryptosporidium, enterotoxigenic Escherichia coli (E. coli), and Shigella.8 In a regional study from Dammam, Saudi Arabia, the commonest organism of diarrhea in children under five years was rotavirus followed by Salmonella, Shigella, Campylobacter jejuni, enteropathogenic E. coli, and non-agglutinating vibrios.9
The detection of the diarrhea-causing organism employs various methods. Polymerase chain reaction is primarily utilized for viruses and specific bacteria. Microscopy serves as a valuable tool for identifying parasites. Meanwhile, stool culture stands as the gold standard for enterobacteria detection, encompassing organisms like Shigella.10
Stool culture, although being the gold standard for bacterial identification and determination of antimicrobial sensitivity, has low sensitivity and poses many technical difficulties.11
Recognizing bacterial causes, especially Shigella, holds significance in averting mortality and minimizing morbidity as emphasized by the World Health Organization (WHO).12 Data suggest that there is increased mortality in children given antibiotics for bloody or non-bloody diarrhea, and the use of antibiotics was proposed to be limited to patients with other comorbidities or children younger than three months.13
The South Al Batinah governorate of Oman has about 476 008 population, mainly low- and middle-income people.14 While bacterial diarrhea is known to occur in low-income areas around the world, there is no data on the prevalence or etiology of bloody diarrhea in the South Al Batinah.15
Our study aimed to determine the occurrence rate and clinical characteristics of children experiencing acute bloody diarrhea associated with Salmonella and Shigella at Rustaq Hospital. Additionally, it sought to outline the antimicrobial sensitivity profile of the stool isolates.
Methods
We conducted a retrospective study of all children under the age of 13 years with bloody diarrhea from 1 June 2019 to 31 June 2023. All children who presented with acute fever and bloody diarrhea seen or admitted in Rustaq Hospital were included in the study. Children > 13 years, known to have other etiological causes of diarrhea, and those who did not fit the definition of acute bloody diarrhea were excluded from the study. The data were retrieved from the Al Shifa electronic database. Demographic data (name, age, sex, nationality, and residence), clinical symptoms (fever, vomiting, abdominal pain, mucus stool, and dehydration status), and laboratory details (C-reactive protein, hemoglobin, leukocytosis, stool red and white blood cell counts, stool culture, and blood culture) were collected. The degree of dehydration was categorized into mild, moderate, or severe. Mild dehydration was defined as when the child had only thirst but normal moist mucous membranes, normal pulse, and normal capillary refill with good urine output. Moderate dehydration was defined as when the child had two of the following signs: restlessness and irritability, reduced tears, deep-set eyes, thirst, and slow return of skin pinch. Severe dehydration was defined as when the child had two of the following signs: lethargy or unconsciousness, sunken eyes, inability to drink or drink poorly, and very slow return of skin pinch.16
Stool samples were collected in a wide-necked sterile container for bacterial culture and microscopy for parasites before commencing antibiotic treatment. MacConkey agar was used in the lab to identify Salmonella–Shigella agar and thiosulfate-citrate-bile salts-sucrose agar. After overnight incubation at 37 °C, the plates were observed for Salmonella and Shigella colonies. For stool bacterial sensitivity, the following antibiotics were tested using the disc diffusion method: ampicillin, ceftriaxone, chloramphenicol, and ciprofloxacin. At times, not all antibiotic discs were available for testing. Only Salmonella and Shigella were cultured due to their management impact in the case of Salmonella typhi and the need for treatment for Shigella. Other bacterial causes such as E. coli and Campylobacter are self-limited diseases that require only supportive care.
The data were analyzed using SPSS (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Numeric values underwent a normality test before analysis. Results were expressed as mean and SD or median with interquartile range. Statistical associations were calculated using the Student’s t-test for continuous data and the chi-square test for categorical data. A p-value < 0.05 was considered statistically significant.
Ethical approval was obtained from the Research Centre in South Al Batinah with research code no. 01062023.
Results
A total of 1160 children were admitted with gastroenteritis at Rustaq Hospital during the study period. Out of the total number, 154 (13.3%) presented with acute bloody diarrhea. One child had missing data. Among these patients, 151 (98.7%) were Omanis, with a male-to-female ratio of 1.3:1. Table 1 demonstrates the demographic data of the population. Most of the patients came from Rustaq, followed by Al Musannah.
Table 1: Demographic data of the study group.
|
Sex
|
|
Male
|
87
|
56.9
|
|
Female
|
66
|
43.1
|
|
Nationality
|
|
Omani
|
151
|
98.7
|
|
Non-Omani
|
2
|
1.3
|
|
Age, years
|
|
Median
|
2
|
IQR = 1–5
|
|
≤ 5
|
128
|
83.7
|
|
≤ 1
|
52
|
34.0
|
|
>5
|
25
|
16.3
|
|
Median weight, kg
|
13.0
|
IQR = 10.0–18.0
|
|
Residence
|
|
Rustaq
|
65
|
42.5
|
|
Al Mussanah
|
34
|
22.2
|
|
Barka
|
21
|
13.7
|
|
As Suwaiq
|
12
|
7.8
|
|
Al Awabi
|
10
|
6.5
|
|
Nakhal
|
8
|
5.2
|
IQR: interquartile range.
The most frequent complaint of the children was watery stools, followed by fever. Table 2 demonstrates the clinical signs, symptoms, and basic blood labs of the patients.
Table 2: Clinical characteristics of patients with bloody diarrhea.
|
Watery stools
|
136
|
88.9
|
|
Fever
|
122
|
79.7
|
|
Vomiting
|
98
|
64.1
|
|
Mucoid stools
|
54
|
35.3
|
|
Bloody stools
|
50
|
32.7
|
|
Abdominal pain
|
30
|
19.6
|
|
Degree of dehydration
|
|
|
|
None
|
59
|
38.6
|
|
Mild
|
57
|
37.3
|
|
Moderate
|
21
|
13.7
|
|
Severe
|
16
|
10.5
|
|
Type of dehydration
|
|
|
|
Eunatremia
|
140
|
91.5
|
|
Hypernatremia
|
2
|
1.3
|
|
Hyponatremia
|
7
|
4.6
|
|
Blood results
|
Value
|
IQR
|
|
Median hemoglobin, gm/dL
|
13.0
|
11.4–12.3
|
|
Median C-reactive protein
|
45.5
|
20.0–126.0
|
|
White blood cell ×109/ mL
|
9.5
|
6.6–13.0
|
HPF: high power field; IQR: interquartile range.
Of the 153 children, 92 (60.1%) had positive stool cultures and 61 (39.9%) had negative stool cultures for either Salmonella or Shigella. Of the children with positive stool culture, 58 (63.0%) had non-typhoidal Salmonella (SNT), and 34 (37.0%) had Shigella infection. No further identification of the Salmonella or Shigella type was required for clinical management purposes.
On a sub-analysis of the population age-wise, in children younger than one year with bloody diarrhea, 41 (78.8%) had fever and only 35 (67.3%) had stool culture positive for bacteria. Thirty-two (61.5%) children had Salmonella growth and three (5.8%) had Shigella in the stool. No significant statistical association was noted between fever and stool bacterial growth (p = 0.676) in children under the age of one year. Only 39 (25.5%) patients were not given antibiotics while the rest of the patients did receive antibiotics.
Bacteremia with blood culture positive for the same organism as isolated from the stool was evident in only three children. All of these children were under the age of one year (10, seven, and eight months), and all had Salmonella growth. The Salmonella-causing bacteremia was sensitive to ceftriaxone, ampicillin, and trimethoprim. All children had a normal leukocyte count. None of the children had further complications from Salmonella bacteremia, and none of them had sickle cell disease.
For children under five years, 55 (43.0%) children had Salmonella isolated and 26 (20.3%) had Shigella isolated. Forty-six (35.9%) children had no growth in stool cultures. In contrast, among children > five years, only three (12.0%) had Salmonella and eight (32.0%) had Shigella.
Regarding antibiotic sensitivity, some patients were not tested for the whole panel of antibiotics sensitivity based on test availability. However, the lowest number of patients tested for antibiotic sensitivity was for ceftriaxone at 41 (70.7%) patients. For the rest of the patients, > 90% of the isolates were tested for the whole panel of antibiotics, except trimethoprim for Shigella, where 29 (85.3%) children were tested. Table 3 demonstrates the pattern of antibiotic sensitivity of isolated Shigella and Salmonella.
Table 3: Antibiotic sensitivity of isolated Salmonella and Shigella.
|
Salmonella, n = 58
|
41 (70.7)
|
53 (91.4)
|
54 (93.1)
|
54 (93.1)
|
|
Sensitive
|
38 (92.7)
|
43 (81.1)
|
43 (79.6)
|
50 (92.6)
|
|
Not sensitive
|
3 (7.3)
|
10 (18.9)
|
11 (20.4)
|
4 (7.4)
|
|
Shigella, n = 34
|
31 (91.2)
|
31 (91.2)
|
32 (94.1)
|
29 (85.3)
|
|
Sensitive
|
4 (12.9)
|
5 (16.1)
|
15 (46.9)
|
5 (17.2)
|
Cipro: ciprofloxacin; Ampi: ampicillin; TMP: trimethoprim.
Thirty-eight (92.7%) children with Salmonella were sensitive to ceftriaxone and 43 (79.6%) were sensitive to ciprofloxacin. The three (7.3%) children with Salmonella resistant to ceftriaxone were all sensitive to ciprofloxacin.
Regarding Shigella, out of the 31 children tested, only four (12.9%) children were sensitive to ceftriaxone. Regarding ciprofloxacin, out of the 32 children tested, only 15 (46.9%) were sensitive while 17 (53.1%) were resistant to ciprofloxacin. Additionally, none of the 17 children with Shigella resistant to ciprofloxacin were sensitive to ceftriaxone.
Overall, 108 (70.6%) children received empirical antibiotics, while 39 (25.5%) did not receive antibiotics. The most common prescription pattern was combined ceftriaxone and metronidazole (n = 43; 28.1%), followed by ceftriaxone or cefotaxime alone. Table 4 demonstrates the pattern of empirical antibiotics used for the patients.
Table 4: Frequency and type of antibiotics used in children with bloody diarrhea upon presentation.
|
Ceftriaxone and metronidazole
|
43 (28.1)
|
|
No antibiotics
|
39 (25.5)
|
|
Ceftriaxone or cefotaxime
|
29 (19.0)
|
|
Metronidazole
|
28 (18.3)
|
|
Amoxy-clavulanic acid
|
8 (5.2)
|
|
Missing
|
6 (3.9)
|
Using analysis of variance, no association was detected with fever, abdominal pain, leukocytosis, CRP, and stool culture positivity for Shigella or Salmonella. However, there was an association noted between stool leukocyte count and stool culture positivity (p = 0.026)
Ten children had a co-infection of Salmonella and Entamoeba histolytica trophozoites, while four had Shigella and amoebic trophozoites. Table 5 demonstrates the isolated parasites detected on stool microscopy.
Table 5: Detection of parasites on stool microscopy from the children with bloody diarrhea.
|
E. histolyica cyst
|
63 (41.2)
|
|
E. histolyica cyst trophozoite
|
29 (19.0)
|
|
Giardia lamblia
|
2 (1.3)
|
|
Hymenolepis nana
|
1 (0.7)
|
|
Taenia species
|
1 (0.7)
|
|
Nil
|
56 (36.6)
|
|
Missing
|
1 (0.7)
|
Regarding the seasonal variation of the incidence of bloody diarrhea, the months from January to July had the most recorded cases, while there was a sharp decline immediately after July until the end of December. This phenomenon was consistent over the years of the study [Figure 1].
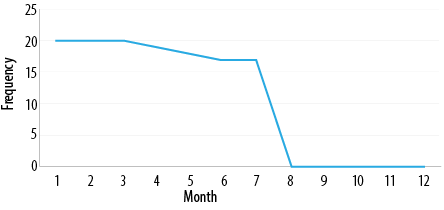 Figure 1: The frequency of bloody diarrhea in Rustaq Hospital during the years of the study.
Figure 1: The frequency of bloody diarrhea in Rustaq Hospital during the years of the study.
Discussion
This study reports a prevalence of 13.2% for bloody diarrhea among children admitted to a regional area with low resources and acute diarrhea. While Shigella has been reported as the most common bacterial pathogen in multiple studies,2–4 in our study, Salmonella was the most common (63.0%) among the whole group particularly in children < five years. Our findings are similar to a study from Brazil on 260 children, which showed that Shigella occurred mostly in children > five years, while Salmonella was more common in younger children.17
The use of antibiotics is recommended mainly for Shigella as it leads to improvement of symptoms within 48 hours and prevents the prolongation of the disease over many weeks.18 Treatment of SNT is mainly supportive except for infants < six months or immunocompromised patients.19 Unlike Shigella, Salmonella is also a self-limiting disease.20
Regarding antibiotic sensitivity, our study demonstrated that 92.7% of the Salmonella isolates were sensitive to ceftriaxone, and nearly 80.0% were sensitive to ampicillin and ciprofloxacin. The low resistance to ceftriaxone is similar to the prevalence reported in the Eastern Mediterranean study, with only about 10% resistance.21 Our study was also in line with the reported resistance to ciprofloxacin for Shigella in the Southeast Asian Region, which rose from 4% to 76% in 2008, close to our Shigella resistance to ciprofloxacin of 53.1%.21
Antibiotic use in cases of Salmonella-related diarrhea is not routinely recommended. Salmonella enterocolitis is a self-limited disease.22 Moreover, antibiotic use in Salmonella infection might trigger hemolytic uremic syndrome and possibly prolong the disease duration.23,24 In our setup, as Salmonella was the most common pathogen in children under the age of one year and few children had bacteremia, it was reasonable to start children under the age of one with either ampicillin or ceftriaxone until stool culture results are available.
In contrast, the situation is reversed with Shigella, where > 83.0% of the isolates were resistant to ceftriaxone and ampicillin, and > 50.0% are resistant to ciprofloxacin. Hence, it is not effective to use any of these medications for Shigella enterocolitis. The WHO recommends ciprofloxacin as an oral medication for ambulatory care for bloody diarrhea in children related to Shigella mainly.12 However, glucose 6-phosphate dehydrogenase deficiency (G6PD) represents an extra obstacle to tackle in our population, as G6PD deficiency reaches 26% of the general population.25 Therefore, in children with G6PD deficiency or of unknown status, macrolides like clarithromycin might be more appropriate26 and needs to be tested in our population.
The children who were stool culture negative (n = 61, 39.9%), even though they had fever and bloody diarrhea, were likely to have another pathogen that was not tested, mainly E. coli and Campylobacter. Acute bloody diarrhea is not commonly attributed to amoebic diarrhea. Amoebic colitis is usually an insidious process and does not present with acute febrile diarrhea. Moreover, it is important to note that Entamoeba histolytica can be easily confused with the normal gut flora of Entamoeba dispar and the low-virulence Entamoeba moshkovskii.27,28
It is interesting that 70.6% of the population received antibiotics regardless of age or clinical condition of the child. Moreover, nearly 50.0% of the patients received metronidazole in combination with cephalosporin or in isolation. The use of metronidazole in not indicated in acute febrile diarrhea. The overuse of antibiotics in general and using inappropriate antibiotics or bloody diarrhea raise concerns on the medial awareness amongst physicians in the institution.
Of interest is the seasonal distribution of bloody diarrhea, as it is almost confined to the first half of the year with no reported cases during the second half. Infective diarrhea is not commonly associated with seasonal changes, and this phenomenon needs to be studied further.
The limitations of the study mainly include the absence of any data on the growth of E. coli or Campylobacter due to the unavailability of testing means in a resource-limited secondary hospital like AGH, particularly as the management is not affected by these organisms. Additionally, there was no testing done for macrolide sensitivity for either Salmonella or Shigella organisms.
Conclusion
Acute bloody diarrhea is a significant problem in our hospital. Fever and mucoid loose stools are the most common symptoms associated with bacterial pathogens. SNT is more common than Shigella in children under the age of five and is sensitive to a wide range of antibiotics. Shigella, on the other hand, is less common but is resistant to multiple antibiotics, including ciprofloxacin. There is significant overuse of antibiotics in children with bloody diarrhea.
Our study advocates for starting antibiotics only after verification of the organism by stool culture, except for children under the age of one year or those who are immunocompromised. G6PD is of concern in our population, and macrolides might be a safer option than fluoroquinolones for the treatment of Shigella.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study
references
- 1. World Health Organization. Diarrhoeal disease. 2024 [cited 2023 December 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
- 2. Muzembo BA, Kitahara K, Mitra D, Ohno A, Khatiwada J, Dutta S, et al. Burden of Shigella in South Asia: a systematic review and meta-analysis. J Travel Med 2023 Feb;30(1):taac132.
- 3. Battikhi MN. Epidemiological study on Jordanian patients suffering from diarrhoea. New Microbiol 2002 Oct;25(4):405-412.
- 4. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013 Jul;382(9888):209-222.
- 5. Patel PK, Mercy J, Shenoy J, Ashwini B. Factors associated with acute diarrhoea in children in Dhahira, Oman: a hospital-based study. Eastern Mediterranean Health Journal 2008;14(3):571-578.
- 6. Ministry of Health, Oman Annual Health Report. 2022. Department of Health Information & Statistics, Ministry of Health. [cited 2024 July 30]. Available from: https://www.moh.gov.om/documents/274609/7264771/Annual+Health+Report+2022/47623227-57f9-d9b7-372b-f16d8af6d91f .
- 7. Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the global burden of disease study 1990-2016. Lancet Infect Dis 2018 Nov;18(11):1229-1240.
- 8. Pernica JM, Steenhoff AP, Welch H, Mokomane M, Quaye I, Arscott-Mills T, et al. Correlation of clinical outcomes with multiplex molecular testing of stool from children admitted to hospital with gastroenteritis in Botswana. J Pediatric Infect Dis Soc 2016 Sep;5(3):312-318.
- 9. Qadri MH, Al-Ghamdi MA, Imadulhaq M. Acute diarrheal disease in children under five years of age in the eastern province of Saudi Arabia. Ann Saudi Med 1990;10(3):280-284.
- 10. da Cruz Gouveia MA, Lins MT, da Silva GA. Acute diarrhea with blood: diagnosis and drug treatment. J Pediatr (Rio J) 2020;96(Suppl 1):20-28.
- 11. Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AK. Shigellosis. Lancet 2018 Feb;391(10122):801-812.
- 12. World Health Organization. The treatment of diarrhea. A manual for physicians and other senior health workers. 2005 [cited 2024 April 23]. Available from: https://www.who.int/publications/i/item/9241593180.
- 13. Mokomane M, Kasvosve I, de Melo E, Pernica JM, Goldfarb DM. The global problem of childhood diarrhoeal diseases: emerging strategies in prevention and management. Ther Adv Infect Dis 2018 Jan;5(1):29-43.
- 14. Ministry of Health. Episodes of diarrhoea among children below 5 years of age. Reported by MOH Health Institutions during 2021. Annual Health Report. 2021 [cited 2024 July 30]. Available from: https://www.moh.gov.om/documents/274609/274947/%D8%A7%D9%84%D8%AA%D9%82%D8%B1%D9%8A%D8%B1+%D8%A7%D9%84%D8%B5%D8%AD%D9%8A+%D8%A7%D9%84%D8%B3%D9%86%D9%88%D9%8A+2021/52125317-99ba-ef59-5e94-3d160840f02d.
- 15. Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 1999;77(8):651-666.
- 16. Burgunder L. The harriet lane handbook. 22nd ed. Fluids and Electrolytes; 2021. p. 261.
- 17. Diniz-Santos DR, Santana JS, Barretto JR, Andrade MG, Silva LR. Epidemiological and microbiological aspects of acute bacterial diarrhea in children from Salvador, Bahia, Brazil. Braz J Infect Dis 2005 Feb;9(1):77-83.
- 18. Williams PC, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr Int Child Health 2018 Nov;38(sup1):S50-S65.
- 19. Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev 2012 Nov;11(11):CD001167.
- 20. Pegues D, Miller S. Salmonella species, including Salmonella typhi. In: Mandell, Douglas and Bennett’s principles and practices of infectious diseases. 7th ed. Philadelphia: Elsevier; 2010. p. 2887-2903.
- 21. Neupane R, Bhathena M, Das G, Long E, Beard J, Solomon H, et al. Antibiotic resistance trends for common bacterial aetiologies of childhood diarrhoea in low- and middle-income countries: a systematic review. J Glob Health 2023 Jul;13:04060.
- 22. Qamar FN, Hussain W, Qureshi S. Salmonellosis including enteric fever. Pediatr Clin North Am 2022 Feb;69(1):65-77.
- 23. Mele C, Remuzzi G, Noris M. Hemolytic uremic syndrome. Semin Immunopathol 2014 Jul;36(4):399-420.
- 24. Sirinavin S, Garner P. Antibiotics for treating salmonella gut infections. Cochrane Database Syst Rev 2000;2(2):CD001167.
- 25. Daar S, Vulliamy TJ, Kaeda J, Mason PJ, Luzzatto L. Molecular characterization of G6PD deficiency in Oman. Hum Hered 1996;46(3):172-176.
- 26. Florez ID, Niño-Serna LF, Beltrán-Arroyave CP. Acute infectious diarrhea and gastroenteritis in children. Curr Infect Dis Rep 2020 Jan;22(2):4.
- 27. Diamond LS, Clark CG. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol 1993;40(3):340-344.
- 28. Ali IK, Hossain MB, Roy S, Ayeh-Kumi PF, Petri WA Jr, Haque R, et al. Entamoeba moshkovskii infections in children, Bangladesh. Emerg Infect Dis 2003 May;9(5):580-584.