Malignant pleural mesothelioma (MPM) is an aggressive malignancy associated with high mortality.1 There are other malignancies that mimic MPM clinically and radiologically, presenting diagnostic challenges.2–6 In this report, we describe a case of a 57-year-old man with a clinical and radiological presentation in keeping with MPM or its mimics. However, pleural histological and immunohistochemistry showed no clear evidence for a specific line of differentiation, making management decisions difficult. His condition was labeled as an undifferentiated epithelioid malignant neoplasm.
Case Report
A 57-year-old Egyptian man presented with a three-month history of left flank pain radiating to the left chest. He had lost 10 kg weight. He was a non-smoker and had no other respiratory symptoms. As an electrical engineer, he worked mainly as a researcher and had no significant exposure to asbestos. There was no other significant medical history, including urinary complaints. He sought medical advice and was investigated for the presence of renal calculi; however computed tomography renal was normal. Clinical examination revealed reduced breath sounds, reduced vocal resonance, and dullness over the left lower chest with no significant lymphadenopathy. Other systemic examination results were unremarkable.
Initial laboratory investigations (electrolytes, liver function test, serum calcium and phosphate, and urine analysis) were unremarkable apart from normocytic normochromic anemia with a hemoglobin level of 10.4 g/dL. The chest X-ray showed blunting of the left costophrenic angle [Figure 1]. High-resolution computed tomography of the chest showed diffuse circumferential nodular pleural thickening encasing the left lung with a mild to moderate pleural effusion and direct invasion of the posterior part of the ninth rib and left pericardium. [Figure 2]. Further characterization by a positron emission tomography (PET) scan showed extensive fluorodeoxyglucose uptake revealing left pleural thickening and entire left hemithorax extending into the adjacent mediastinum. There was a bony erosion of this mass onto the posterior aspects of the left ninth rib and the transverse process of the T9 vertebra. There were multiple FDG-avid retroperitoneal lymph nodes with a maximum standard unit value of 8.7. The left adrenal gland was nodular and demonstrated increased FDG uptake (standard unit value = 4.6) [Figure 3].
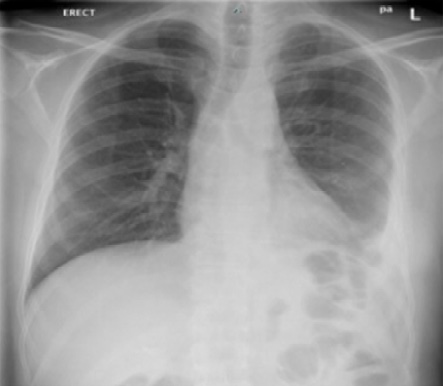 Figure 1: Chest X-ray shows left costophrenic angle blunting.
Figure 1: Chest X-ray shows left costophrenic angle blunting.
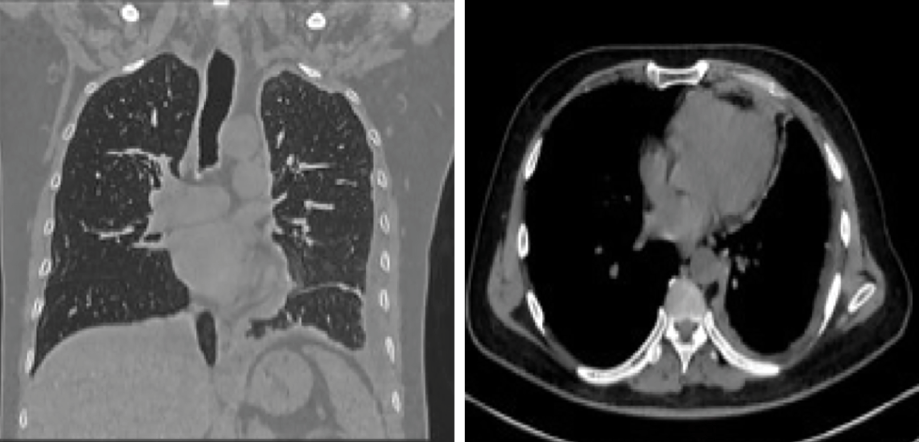 Figure 2: The chest's high-resolution CT shows diffuse circumferential nodular pleural thickening encasing the left lung with a mild to moderate pleural effusion and direct invasion of the left pericardium.
Figure 2: The chest's high-resolution CT shows diffuse circumferential nodular pleural thickening encasing the left lung with a mild to moderate pleural effusion and direct invasion of the left pericardium.
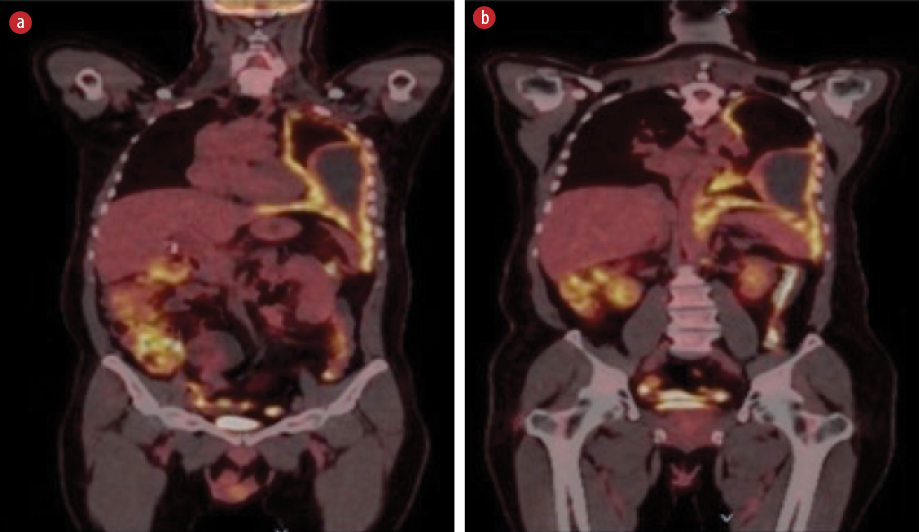 Figure 3: (a) Extensive circumferential nodular thickening is seen due to significant fluorodeoxyglucose (FDG) uptake involving the entire left hemothorax extending into the adjacent mediastinum. (b) Shows FDG-avid retroperitoneal lymph nodes.
Figure 3: (a) Extensive circumferential nodular thickening is seen due to significant fluorodeoxyglucose (FDG) uptake involving the entire left hemothorax extending into the adjacent mediastinum. (b) Shows FDG-avid retroperitoneal lymph nodes.
Video-assisted thoracoscopic surgery revealed hemorrhagic pleural fluid, nodular thickening of the pleura with high vascularity, and nodules over the left diaphragm. Multiple pleural biopsies were taken. Pleural fluid cytology revealed many lymphocytes, some neutrophils and eosinophils, and occasional atypical cells. Tuberculosis GeneXpert was negative from the biopsy sample. No mycobacteria were isolated from the culture. Histopathology showed that the tissue was infiltrated by predominantly epithelioid cells with pale cytoplasm and large atypical vesicular nuclei with prominent nucleoli. These tumor cells were associated with a variable inflammatory infiltrate, including prominent histiocytes. Many mitotic figures, including atypical forms, were seen [Figure 4].
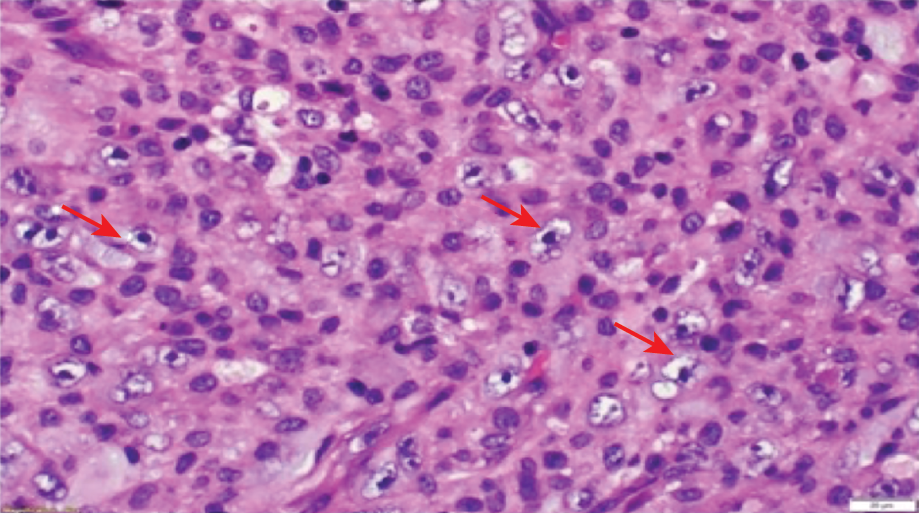 Figure 4: The specimen shows tissue infiltrated by predominantly epithelioid cells with pale cytoplasm and large atypical vesicular nuclei with prominent nucleoli (arrows). These tumor cells are associated with
Figure 4: The specimen shows tissue infiltrated by predominantly epithelioid cells with pale cytoplasm and large atypical vesicular nuclei with prominent nucleoli (arrows). These tumor cells are associated with
a variable inflammatory infiltrate including prominent histiocytes. Hematoxylin and eosin stain (magnification = 60 ×).
The atypical cells were negative for the typical immunohistochemical markers, pan-keratin, CAM 5.2, cytokeratin (CK)5/6, calretinin, WT1, D2-40, CK7, CK20, CDX2, thyroid transcription factor-1 (TTF1), Napsin A, smooth muscle actin, desmin, Melan-A, inhibin, ALK1, CD34, BER-EP4, epithelial membrane antigen, renal cell carcinoma (RCC), prostate-specific antigen, CD10, S100, leukocyte common antigen, CD3, CD20, CD15, CD30, PAX5, multiple myeloma oncogene 1, CD21, CD23, BCL2, BCL6, CD138, Epstein-Barr Virus Early RNA-in situ hybridization, CD1a, MyoD1, myogenin, placental alkaline phosphatase, MPX CD117, MDM2, CDK4, PU.1, CD163, claudin-4, CD34, SALL4, ERG, and CD35. SMARCA4 immunostain was retained in the tumor cells. Fluorescence in situ hybridization for MDM2 gene amplification was negative. As no evidence of a specific line of differentiation emerged, the disease was described as an undifferentiated epithelioid malignant neoplasm.
We managed the patient symptomatically with analgesia before the final pathology findings were released. He then traveled abroad to be treated in his own country. Unfortunately, he passed away as his undifferentiated epithelioid malignant neoplasm behaved aggressively.
Discussion
Our patient’s presentation and imaging findings mimicked those of MPM, a rare aggressive malignancy with high mortality.1 Typically, MPM patients present with a history of significant past exposure to asbestos for decades.2 Radiological findings include diffuse unilateral pleural thickening, mass or large pleural effusion, pleural plaque, or calcification.2
Some malignancies may mimic MPM clinically and radiologically, including sarcomas, pleural metastasis particularly lung adenocarcinoma, tuberculosis pleurisy, and diffuse large B-cell lymphoma.3–7 Some of these tumors can directly invade the serosal membrane and behave like mesothelioma, presenting a diagnostic challenge. In our patient, MPM could be ruled out because all the MPM mesothelial immunostains characteristics (CK7, CK5/6, WT1, calretinin, and D2-40) were negative.
Differentiating MPM from other malignant neoplasms or benign reactive processes is still an evolving subject. Generally, the presence of an invasion with features such as in our patient (e.g., invasion to the lung or ribs) means the benign process is very unlikely.8 Sarcomatoid carcinoma involving the pleura is an extremely rare malignancy and tends to be very aggressive with rapid invasion. There are few case reports of pulmonary sarcomatoid carcinoma mimicking MPM; however, unlike in our patient, all cases of sarcomatoid carcinoma had the histopathological examination of the biopsy showing positive stains for CK7, AE1/AE3, and vimentin.9–11
Lung adenocarcinoma commonly presents with malignant pleural effusion, its hallmark in histopathological examination is positive TTF-1 and Napsin A immunohistochemistry stains, which help to confirm the diagnosis of lung primary. Other markers such as claudin-4, MOC31, BG8 (LewisY), and BER-EP4 have high yields of the same diagnosis.12 In the present case, many of these immunohistochemical markers (TTF-1, Napsin A, claudin-4, and BER-EP4) came out negative, ruling out the possibility of lung adenocarcinoma.
One of the important differential diagnoses of MPM is RCC, as there is 1–2% of malignant pleural effusions diagnosed eventually as metastatic RCC.13 Usually, patients with RCC have positive stains of PAX8, PAX2, claudin-4, and CD15 in their histopathological examination.12 In our case, RCC was ruled out with negative staining for cytokeratin markers, RCC, CD10, claudin-4, and CD15. Lymphoma was excluded due to negative hematolymphoid immunohistochemistry (leukocyte common antigen, CD3, CD20, CD15, CD30, PAX5, multiple myeloma oncogene 1, CD21, CD23, BCL2, BCL6, and CD138).
Conclusion
Histological and immunohistochemical examinations of our patient’s biopsy did not show any positivity for MPM or its common mimics. There was no clear evidence of a specific line of differentiation, which made the treatment very difficult. Therefore, it was labeled as an undifferentiated epithelioid malignant neoplasm.
Disclosure
The authors declared no conflicts of interest. Informed consent was obtained from the patient.
references
- 1. Odgerel CO, Takahashi K, Sorahan T, Driscoll T, Fitzmaurice C, Yoko-O M, et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup Environ Med 2017 Dec;74(12):851-858.
- 2. Janes SM, Alrifai D, Fennell DA. Perspectives on the treatment of malignant pleural mesothelioma. N Engl J Med 2021 Sep;385(13):1207-1218.
- 3. Szulkin A, Szatmári T, Hjerpe A, Dobra K. Chemosensitivity and resistance testing in malignant effusions with focus on primary malignant mesothelioma and metastatic adenocarcinoma. Pleura Peritoneum 2016 Sep;1(3):119-133.
- 4. Shinohara T, Shiota N, Kume M, Hamada N, Naruse K, Ogushi F. Asymptomatic primary tuberculous pleurisy with intense 18-fluorodeoxyglucose uptake mimicking malignant mesothelioma. BMC Infect Dis 2013 Jan;13(1):12.
- 5. Dawood W, La Garza Henriette D, Iliescu G, Moran C, Grosu H. Pleural tuberculosis mimicking malignant mesothelioma. Respir Med Case Rep 2019 Nov;29:100964.
- 6. Xu T, Wang L, Zhou L, Wang Z, Zhu Y, Ju F. Primary colorectal diffuse large B-cell lymphoma initially presenting with pleural effusion: report of one case and review of literature. Int J Clin Exp Pathol 2020 Feb;13(2):254-260.
- 7. Ito T, Fujita K, Okamura M, Okuno Y, Saito Z, Kanai O, et al. Pleural involvement of diffuse large B-cell lymphoma mimicking malignant pleural mesothelioma. Respir Med Case Rep 2020 Aug;31:101206.
- 8. Romei C, Fanni SC, Volpi F, Milazzo A, D’Amore CA, Colligiani L, et al. New updates of the imaging role in diagnosis, staging, and response treatment of malignant pleural mesothelioma. Cancers (Basel) 2021 Aug;13(17):4377.
- 9. Inomata M, Kawagishi Y, Yamada T, Miwa T, Hayashi R, Kashii T, et al. [Two cases of pulmonary sarcomatoid carcinoma mimicking malignant mesothelioma]. Nihon Kokyuki Gakkai Zasshi 2010 Jan;48(1):33-38.
- 10. Zhao S, Hou T, Li Q. Sarcomatoid carcinoma of the pleura: a case report. Radiol Infect Dis 2019;6(3):128-132.
- 11. Ciarallo A, Makis W, Novales-Diaz JA, Lisbona R. Sarcomatoid carcinoma (carcinosarcoma) of the lung mimics malignant pleural mesothelioma on 18F-FDG PET/CT: a report of 2 cases. Clin Nucl Med 2012 Apr;37(4):416-419.
- 12. Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med 2018 Jan;142(1):89-108.
- 13. Arain A, Swaminathan M, Kumar J. Renal cell carcinoma presenting as pleural effusion. WMJ 2019 Apr;118(1):49-51.