|
Introduction
Urinary bladder cancer is the sixth most common cancer worldwide and the second most common malignancy of the genitourinary tract after prostate cancer, and represents a heterogeneous group of neoplasms. The natural history of these bladder cancers is that of recurrence and progression to higher grades and stages.1 Urothelial (transitional cell) carcinoma is by far the most frequent type of bladder cancer.2 Bladder tumors are more common in industrial areas and their incidence is increased with exposure to cigarette smoking and arylamines.3 Schistosoma hematobium is thought to be pathogenetically related to squamous cell carcinoma (SCC) as well as transitional cell carcinoma of the bladder. This is in accordance with the high prevalence of this type of cancer in areas of the world infested with this parasite.4
The clinical significance of bladder tumors depends upon their histological grade, differentiation and most importantly, on the depth of invasion of these lesions. Both tumor grade and stage of urothelial carcinoma are highly correlated with recurrence, progression and patient survival rates.2 No uniformly grading system for bladder cancer currently exists. The most commonly used systems are based on the degree of anaplasia of the tumor cells.5 In 1998, the World Health Organization and the International Society of Urologic Pathology (WHO/ISUP) decided to classify many of these tumors as urothelial neoplasms.
This WHO/ISUP system was an attempt to develop a broad consensus in the classification of urothelial neoplasms, building upon earlier works and classification systems. It was meant to serve as a springboard for future studies that will help refine this classification, thus enabling us to provide better correlation of these lesions with their biologic behavior using uniform terminology.6 The assessment of urinary cytology is helpful in urothelial malignancy screening tests and is sufficient for patient follow-up as well as control of any residual tumor.7 The Republic of Yemen is a large country with various climatic, topographic and environmental conditions. Its provinces are characterized by different social and genetic patterns. Until now, this country lacks a National Cancer Registry Center (NCRC), hence there is shortage of cancer information and reliable data.
This study aims to highlight the clinicopathological features of urinary bladder cancer in Yemen, and to describe the histological grading of urothelial neoplasms according to the World Health Organization and International Society of Urologic pathology (WHO/ISUP 1998) classification.
Methods
A descriptive record based study of 316 cases of urinary bladder cancers was conducted at the Department of pathology, Faculty of Medicine and Health Sciences, Sana'a University, during the period from 1st January 2005 to 30th April 2009. The diagnosis of neoplasm was made primarily in private laboratories of two consultant pathologists in Sana'a, who received a total of 316 cases; 302 cystoscopic biopsies, 7 transuretheral resections, 6 radical cystectomy, and one partial cystectomy (from Sana'a and other Yemeni provinces). Most of the patients were referred to Sana'a for further investigations and therapy, where most of the histopathologists and oncologists are practicing.
The biopsies were fixed in 10% formalin solution before being processed by manual and automatic tissue processor (Shandon Southern product, England, Cheshire). After embedding in paraffin blocks, several thin sections of 2-3 micrometer thickness were cut from each block. The sections were stained with hematoxylin and eosin stains for routine histological diagnosis. During histological analysis, the samples were evaluated based on the papillary configuration, cytological characters, invasion of lamina propria and muscle, inflammation, schistosomal lesions, metaplasia, dysplasia; and the urothelial neoplasms were then categorized according to the WHO/ISUP1998 classification.6
The present study focuses on cases that revealed a clear histological picture of urinary bladder neoplasms on light microscope, and excludes the doubtful cases of suggestive urinary bladder neoplasm, as well as cases revealing inadequate material of neoplasm. The medical services in Yemen are spanned between the ministry of health and private hospitals. Furthermore, adequate methods of follow-up are far from optimal because there is no real university hospital where you can be in touch with your clinician colleagues.
Ethical clearance was obtained from the Ethics Committee of the Faculty of Medicine and Health Sciences at Sana'a University. Statistical analysis was performed using the Statistical Package for Social Sciences version 15 (SPSS Inc., Chicago, IL, USA) to calculate Chi square and p-value. A p-value less than 0.05 was considered statistically significant.
Results
The total number of urinary bladder cancers was 316 cases, accounting for 65% of the total urinary bladder biopsies during the period of study. There were 248 (78%) urothelial (transitional) neoplasms with a mean age of 59 years, as well as 53 (17%) squamous cell carcinoma (SCC) with a mean age of 55 years, and 7 (2%) adenocarcinoma with a mean age of 49 years. The remaining types are shown in Table 1. Thirty one (59%) out of 53 cases of SCC observed in this study showed histological evidence of schistosomal eggs and 22 (42%) cases were negative of any evidence suggestive of schistosomal eggs in histological sections.
Out of the 248 urothelial neoplasms, 202 (81%) were males and 46 (19%) were females, with a male to female ratio of 4:1 (p<0.001) and a distinct male preponderance in all histological grades. The grades of urothelial neoplasms according to the WHO/ISUP are presented in Table 2 and demonstrated in Figs. 1 to 3. The age distribution of the 217 cases of urothelial neoplasms is shown in Table 3. The maximum number of cases was encountered in the age group of 61-70 years (n=60; 28%) and the minimum was encountered in the age group of ≤30 years (n=8; 4%). Age range was 12-95 years with a mean age of 60 years for males and 58 years for females.
Table 1: Distribution of urinary bladder cancers with mean and age range (n=316).
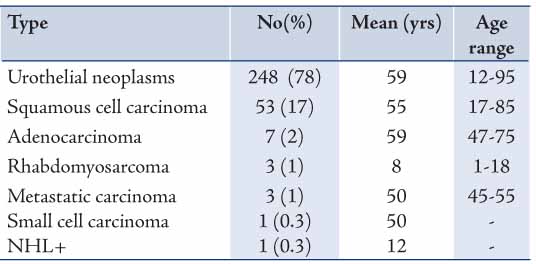
Table 2: Gender distribution of urothelial neoplasm subtypes*.
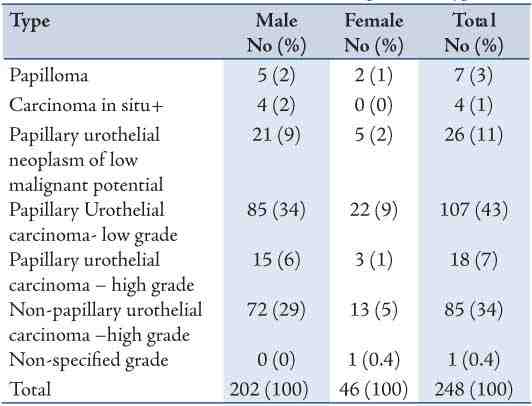
Figure 1: Papillary Urothelial neoplasm of low malignant potential: Note the papillary configuration, normal orientation of the urothelial cells, absent of nuclei pleomorphism and mitotic figures. (Hematoxylin and eosin stain × 400)
Table 3: Age distribution for urothelial neoplasms.

Figure 2: Papillary urothelial carcinoma of low grade: Well-formed and fused papillae that lined by disoriented focally condensed urothelial cells. Note focal pleomorphism and several mitoses (arrow). (H&E stain × 400)
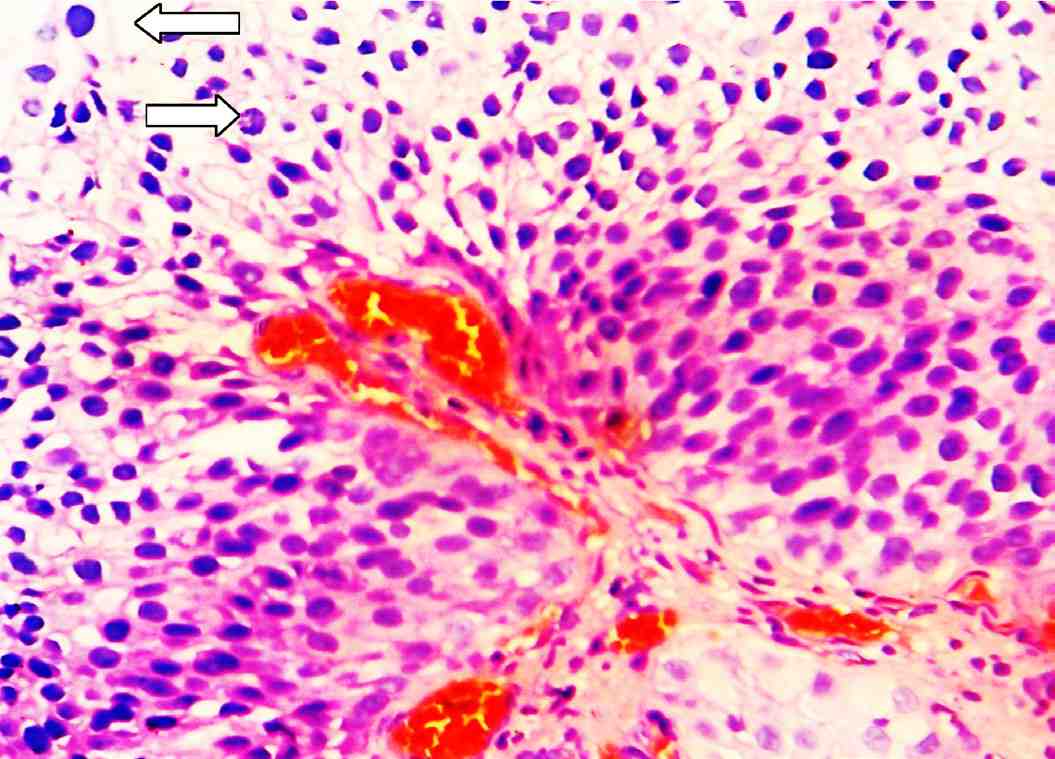
Figure 3: Papillary urothelial carcinoma of high grade: There is significance disturbance of the architecture and the cells have pleomorphic and hyperchromatic nuclei. There are numerous mitotic figures scattered throughout the urothelium (arrows). (H&E stain × 400).
Discussion
Urinary bladder cancer is the sixth most common cancer worldwide, and presents as the second most common malignancy affecting the genitourinary tract after prostate cancer and represents a heterogeneous group of neoplasms.1 Research on urinary bladder cancer in Middle Eastern countries is scarce, and markedly so in Yemen. Ninety-nine percent of bladder neoplasms in this research arose from the epithelium; the most common subtypes were urothelium neoplasms which account for 78% of cases, and SCC which represents 17% of cases. The observed figure for urothelial neoplasms (78%) was markedly similar with that reported from neighboring Saudi Arabia (77%).8 However, lower figures have been reported in Africa; Nigeria (42%),9 and Tanzania (28%).10
In developed countries, over 90% of the bladder cancer cases are urothelium neoplasms with SCC, adenocarcinoma, and rare types of bladder cancer comprising the remaining 10%.11 In the USA, a high frequency of urothelial neoplasms (98%) has been reported by Schned et al.2 In the current study, Squamous cell carcinoma accounted for 17%. However, considerable variability was noted in the prevalence of SCC of the bladder in different parts of the world. It accounted for only 1% of bladder cancers in England,11 and 7% in the United States,12 but as high as 75% in Egypt.13 Approximately 59% of SCC in this study were associated with chronic infection by schistosoma hematobium. An earlier study conducted in Egypt showed that around 80% of SCC were accompanied by chronic infection with schistosoma hematobium.14 Recently, transitional cell carcinoma has become the most frequent type encountered in Egypt due to the significant changes in the etiology of bladder cancer.15 Also, adenocarcinoma (primary bladder, urachal or metastatic) represents 3% of malignant bladder tumors in this study which is similar to what has been reported by other authors.16
In general, it may be said that while comparing the frequency of histological subtypes in the present study with others studies, a clear difference was observed. Such a divergence could be explained in terms of diagnostic approach and/or probably due to the combined effects of environmental and hereditary factors. Additionally, tobacco use is believed to be similarly spread worldwide and it may explain the overall increase in urothelium neoplasms in our patients. Both tumor grade and stage of urothelial carcinoma are highly correlated with recurrence, progression, and patient survival rates.2 The WHO/ISUP grading of urothelial neoplasms of the bladder is of great prognostic significance. The findings shown in Table 2 reveal the distribution of urothelial neoplasm grading according to the WHO/ISUP criteria; carcinoma in situ (2%), papilloma (3%), PUNLMP (11%), PUC-LG (43%), PUC-HG (7%), and Non-PUC-HG (34%). In Jordan, Matalka et al.17 reported 60% of low grade and 40% of high grade. A recent report from the USA2 showed CIS, papilloma, PUNLMP, PUC-LG, PUC-HG and NPUC-HG to be 6%, 0.3%, 26%, 35%, 23%, and 10%, respectively. While in Australia, Samaratunga et al.18 reported 2% papilloma, 22% low malignant potential, 13% low grade, and 22% high grade carcinoma. The variation found between these results could be explained in terms of diagnostic approach and/or techniques applied, number of patients studied, as well as geographical and immunological differences.
The histological grading suffers from all the drawbacks of a subjective evaluation, especially when performed in biopsy material. Furthermore, the differences of a given neoplasm may vary from area to area, thus a cystoscopic biopsy may show a low-grade malignancy as opposed to what is present in the surgical specimen. The paucity of CIS cases in this study was due to the exception of the cases that were seen to be in association with conventional urothelial carcinoma and were especially common in high-grade lesions.
Also in this study, men are nearly 4 times more likely to be effected with bladder urothelial neoplasms than women. Worldwide, the male-to-female ratio ranges between 1:3 and 1:5.19,20 However, a higher ratio was documented in Jordan; 1:9.17 A marked male preponderance was seen in all types in this study as well as worldwide, probably because boys and men are more involved in agricultural and industrial activities making them more exposed to carcinogenic factors. The age of patients ranged from 12 to 95 years with a mean age of 59 years and most of the cases of urothelial neoplasms (70%) were present in patients aged over 51 years and in approximately 30% of younger adults and children. The frequency of urothelial neoplasms in Yemen is increased with increasing age, and a significant difference was observed among the age groups. In the current study, the cases involving younger adults and children showed urothelial neoplasms of predominantly low grade with a favorable clinical outcome, while high grade was encountered in older age groups. These findings are in accordance with those reported in other investigations.11,21 The youngest patient was a 12 year-old boy with urothelial papilloma. However, urothelial neoplasms in patients aged younger than 20 years are generally rare. Fine et al. identified 23 patients with urothelial neoplasms with a mean age of 13 years.21
However, since the current study was limited to recorded data, many difficulties were encountered in the interpretation of results due to incomplete information from patient history, staging and management of the neoplasms. This could not be denied as in all retrospective studies particularly in a country like Yemen, where the medical services are spanned among ministry of health and private hospitals. However, the figures obtained in this study are of significance in making acceptable conclusions on the national level of frequency of urinary bladder cancer.
Conclusion
This study documents a high frequency of urothelial neoplasms, mostly PUC-LG, and Non-PUC-HG with a male preponderance and peak incidence in the 6th decade of age. Future studies are needed to determine in greater detail the risk factors that increase inflammation of the bladder and examine genetic susceptibility of inflammation and markers of inflammation prior to cancer diagnosis. Understanding the role of inflammation may provide important insight on how to reduce bladder cancers worldwide.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Lopez-Beltran A. Bladder cancer: clinical and pathological profile. Scand J Urol Nephrol Suppl 2008 Sep;218(218):95-109.
2. Schned AR, Andrew AS, Marsit CJ, Kelsey KT, Zens MS, Karagas MR. Histological classification and stage of newly diagnosed bladder cancer in a population-based study from the Northeastern United States. Scand J Urol Nephrol 2008;42(3):237-242.
3. Anton-Culver H, Lee-Feldstein A, Taylor TH. Occupation and bladder cancer risk. Am J Epidemiol 1992 Jul;136(1):89-94.
4. Fukushima S, Asamoto M, Imaida K, el-Bolkainy MN, Tawfik HN, Ito N. Comparative study of urinary bladder carcinomas in Japanese and Egyptians. Acta Pathol Jpn 1989 Mar;39(3):176-179.
5. Messing EM. Urothelial tumors of the urinary tract. in: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors.Campbell's Urology. 8th ed. Philadelphia (PA): WB Saunders; 2002; p2732-2784.
6. Epstein JI, Amin MB, Reuter VR, Mostofi FK; Bladder Consensus Conference Committee. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol 1998 Dec;22(12):1435-1448.
7. Ahmed HG, Tom MA. The consequence of delayed fixation on subsequent preservation of urine cells. Oman Med J 2011 Jan;26(1):14-18.
8. Kattan S, Yousef A, Onuora V, Patil M, Al-Jasser A, Al-Ariyan R. The clinicopathological features of bladder carcinoma among Saudis in Riyadh Central Hospital. Ann Saudi Med 1994 Mar;14(2):114-116.
9. Obafunwa JO. Histopathological study of vesical carcinoma in Plateau State, Nigeria. Eur J Surg Oncol 1991 Oct;17(5):489-491.
10. Kitinya JN, Laurèn PA, Eshleman LJ, Paljärvi L, Tanaka K. The incidence of squamous and transitional cell carcinomas of the urinary bladder in northern Tanzania in areas of high and low levels of endemic Schistosoma haematobium infection. Trans R Soc Trop Med Hyg 1986;80(6):935-939.
11. Lynch CF, Cohen MB. Urinary system. Cancer 1995 Jan;75(1)(Suppl):316-329.
12. Costello AJ, Tiptaft RC, England HR, Blandy JP. Squamous cell carcinoma of bladder. Urology 1984 Mar;23(3):234-236.
13. El-Bolkainy MN, Mokhtar NM, Ghoneim MA, Hussein MH. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer 1981 Dec;48(12):2643-2648.
14. Felix AS, Soliman AS, Khaled H, Zaghloul MS, Banerjee M, El-Baradie M, et al. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes Control 2008 May;19(4):421-429.
15. Gouda I, Mokhtar N, Bilal D, El-Bolkainy T, El-Bolkainy NM. Bilharziasis and bladder cancer: a time trend analysis of 9843 patients. J Egypt Natl Canc Inst 2007 Jun;19(2):158-162.
16. Rosai J. Bladder and male urethera. In: Akerman's surgical pathology 8th ed. New York (NY): Mosby; 1996. p.1185-1217.
17. Matalka I, Bani-Hani K, Shotar A, Bani Hani O, Bani-Hani I. Transitional cell carcinoma of the urinary bladder: a clinicopathological study. Singapore Med J 2008 Oct;49(10):790-794.
18. Samaratunga H, Makarov DV, Epstein JI. Comparison of WHO/ISUP and WHO classification of noninvasive papillary urothelial neoplasms for risk of progression. Urology 2002 Aug;60(2):315-319.
19. Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology 2000 Mar;55(3):368-371.
20. Cheng L, Neumann RM, Nehra A, Spotts BE, Weaver AL, Bostwick DG. Cancer heterogeneity and its biologic implications in the grading of urothelial carcinoma. Cancer 2000 Apr;88(7):1663-1670.
21. Fine SW, Humphrey PA, Dehner LP, Amin MB, Epstein JI. Urothelial neoplasms in patients 20 years or younger: a clinicopathological analysis using the world health organization 2004 bladder consensus classification. J Urol 2005 Nov;174(5):1976-1980.
|