|
Abstract
Objective: To evaluate the sensitivity and specificity of Calretinin and Carcinoembryonic antigen as immunocytochemical markers in distinguishing mesothelial cells from metastatic adenocarcinoma cells in effusion cytology.
Methods: This study included 50 patients who presented with effusions (26 pleural and 24 peritoneal), at Al-Kadhimya Teaching Hospital who were selected according to their preliminary diagnosis from 1st December 2010 to 30th June 2011. Effusion fluids were aspirated and processed for both conventional cytological methods using Papanicolaou-stain and immunocytochemical staining with anti Calretinin and Carcinoembryonic antigen.
Results: The sensitivity of cytology for detection of malignant cells was 77%, with 100% specificity and 86% accuracy. Calretinin was observed to be a specific (100%) and sensitive (90%) marker for mesothelial cells (of benign etiology). Carcinoembryonic antigen exhibited 70% sensitivity and 100% specificity for adenocarcinoma cells. When the results of both cytology and immunocytochemistry were considered in conjunction, the sensitivity for the detection of malignancy increased to 97%, with 100% specificity and 98% accuracy.
Conclusion: Calretinin and Carcinoembryonic antigen were found to be useful markers for differentiating reactive mesothelial cells from metastatic adenocarcinoma cells in smears prepared from body fluids. Also, the combination of both cytology and immunocytochemical studies using the two markers can greatly enhance the diagnostic accuracy, sensitivity and specificity in malignant effusions.
Keywords: Calretinin; Carcinoembryonic antigen; Adenocarcinoma; Mesothelial Cells; Effusion.
Introduction
Cytological evaluation of serous effusions poses difficulties on pathologists. Various ancillary studies have been used to increase the diagnostic accuracy of cytology. Immunocytochemical analysis is the most commonly used special technique and often involves the use of a panel of antibodies. Evaluation of effusion cytology is one of the most challenging areas of diagnostic cytopathology.1 Serous effusions are common clinical syndromes that can be divided into benign and malignant. Differentiation between the two kinds of effusions is very important for diagnosis, treatment and prognostic evaluations.2 Although most cases of effusion cytology can be diagnosed on routine cytological preparations, it is often very difficult to make unequivocal interpretation in some cases.3,4
The most common difficulty encountered by cytopathologists worldwide is the inability to separate without dispute the exfoliated atypical benign mesothelial cells from metastatic cells of adenocarcinoma in effusion.5 The reason being that benign mesothelium undergoes myriad architectural and cellular alterations in reaction to numerous stimuli; while on the other hand, well-differentiated or borderline malignant cells can masquerade as benign cells.6 Thus, definitive cytological diagnosis of serous effusions is sometimes unattainable on cytomorphologic grounds alone, and ancillary studies are needed in such instances; and over the last decade, it has become clear that of all the available methods, immunocytochemical stains are superior in the diagnostic workup of effusion cytology.7
This study aims to evaluate the sensitivity and specificity of Calretinin (CAL) and Carcinoembryonic antigen (CEA) as immunocytochemical markers in distinguishing mesothelial cells from metastatic adenocarcinoma cells in pleural and peritoneal effusions with equivocal cytomorphological findings using routine Papanicolaou staining.
Methods
This study was conducted on all patients who presented with effusions (26 pleural and 24 peritoneal) at Al-Kadhimya Teaching Hospital during the study period (from 1st December 2010 to 30th June 2011). The patients were selected on the basis of their preliminary diagnosis from whom effusion fluids were aspirated. Pleural fluid was obtained by thoracentesis,8 and peritoneal fluid by paracentesis.9 The final diagnosis was confirmed depending on clinical history, imaging studies, cytological features and histopathological examinations (considered to be the gold standard).
All clinical information pertaining to age, sex, present and past medical or surgical history were recorded and an absolute confidentiality of the patients’ vital information was maintained for ethical purposes. Ethical approval was obtained from Al-Nahrain Medical college and Teaching Hospital, where the study was conducted.
Five milliliter of fluid samples was poured into a centrifuge tube, and centrifuged at (1500 rpm) for 10 minutes. The supernatant was discarded and the sediment, after tipping the bottom of the tube several times, was smeared on four slides, two of them were ordinary glass slides for conventional cytological examination using Papanicolaou-stain, and the other two slides were positive charged slides for immunocytochemical staining with CAL and CEA using Flex Monoclonal Mouse Anti-Human Calretinin (ready to use) of Clone: DAK-Calret 1, Isotope: IgG1, kappa, Code No.: IS627 (DaKoCytomation, Denmark) and Monoclonal Mouse Anti-Human Carcinoembryonic Antigen of Clone: 11-7, Isotope: IgG1, Kappa, Code No.: M7072 (DaKoCytomation, Denmark), respectively, with Immunoperoxidase secondary detection kit (DakoCytomation kit LSAB2 System-HRP, code K0679) manufactured by DAKO, Denmark in accordance with the manufacturer's instructions.
For antigen retrieval, slides were placed in a plastic jar filled with 250 ml of target solution. The jar was then placed in a microwave oven together with two other jars containing distilled water as a triangle to balance the microwave’s power. They were heated for 10 minutes at 680 watt power, then for 15 minutes at 340 watt power, after which they were left to cool for 20 minutes at room temperature.
The application of primary antibody (CAL was ready to use, and CEA was diluted at a range of 1:25-1:50) was done for one hour at room temperature, then the humid chamber was placed overnight in the refrigerator. Positive control was performed using antigen rich specimen. Sections from apparently normal colon taken from autopsy in which ganglion cells were used as positive control for CAL, and sections from colorectal adenocarcinoma were used for CEA according to the manufacturer's instructions.
Technical negative control was performed by omitting the addition of primary monoclonal antibody and replacing it with buffer saline. As an evaluation of immune staining, a positive stain was indicated by a brown colored precipitate in the following manner: 1) cells labeled by CAL displayed cytoplasmic and nuclear staining; and 2) cells labeled by CEA displayed a cytoplasmic staining pattern. Immunocytochemical reactivity was evaluated by a semiquantative method as follows: a). Intensity of the staining: 0 = no staining, 1 = mild but unequivocal staining, 2 = definite staining of moderate intensity, 3 = strong staining; and b). The immune staining for CAL and CEA was considered positive when unequivocal staining was observed in at least 20% of cells.5,10
After routine cytological evaluation, the cases were categorized as reactive, suspicious and malignant. The diagnoses were confirmed by clinical and histopathological correlation and then selecting 20 benign and 30 metastatic adenocarcinoma effusions.
Data analysis was done using SPSS version 16 (statistical package for social sciences) and Microsoft office Excel 2007. Continuous variables were presented as mean ± standard error of mean, while discrete variables were presented as number and percentage. Sensitivity, specificity, and overall accuracy measurements with positive and negative predictive values from cytology and immunocytochemistry were determined using the following equations:
1. Sensitivity = (TP/ (TP+FN)) × 100
2. Specificity = (TN/ (TN+FP)) × 100
3. Overall accuracy = ((TP+TN)/ (TP+TN+FP+FN)) × 100
4. Positive predictive value = (TP/ (TP+FP)) × 100
5. Negative predictive value = (TN/ (TN+FN)) × 100
TP= true positive, TN= true negative; FN= false negative, FP= false positive
Results
Fifty patients who presented with effusions were enrolled into this prospective study. Twenty-six (52%) samples were pleural effusion fluids and 24 (48%) were ascitic fluids. The mean age of patients who presented with effusions was 56 ± 2 years, with a range of 24 to 75 years. The highest number of patients who presented with effusions was in the age group >60 years (53%). Of the 50 patients with effusions, 26 (52%) patients were females and 24 (48%) were males with a male to female ratio of 0.9:1. Effusions were described as hemorrhagic in 18 (36%) samples and non-hemorrhagic in 32 (64%) samples, with the most common cause of hemorrhagic effusions being malignancy which was present in 16 (89%) out of all the hemorrhagic fluids, and only two (11%) were reactive effusions due to pulmonary embolism and pneumonia (Fig. 1). Out of the 30 malignant effusions with metastatic adenocarcinomas, 16 (53%) cases were hemorrhagic and 14 (47%) were not (Fig. 2).
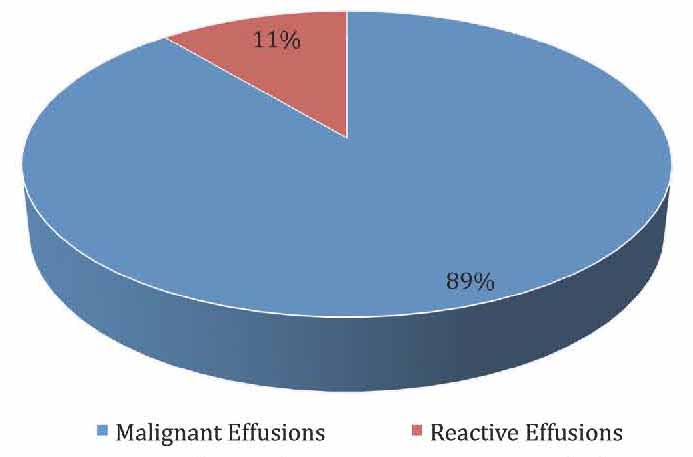
Figure 1: Cause of hemorrhagic effusions in cases studied.
The 50 cases were classified morphologically into reactive, metastatic adenocarcinomas and suspicious for malignancy. Twenty-one (42%) cases were reactive effusion (RE) showing reactive mesothelial cells (RMC) presenting singly or as berry-like clusters with scalloped contours, with round nuclei and smoothly contoured membranes. The cells had low nuclear to cytoplasmic ratio as shown in Fig. 3.
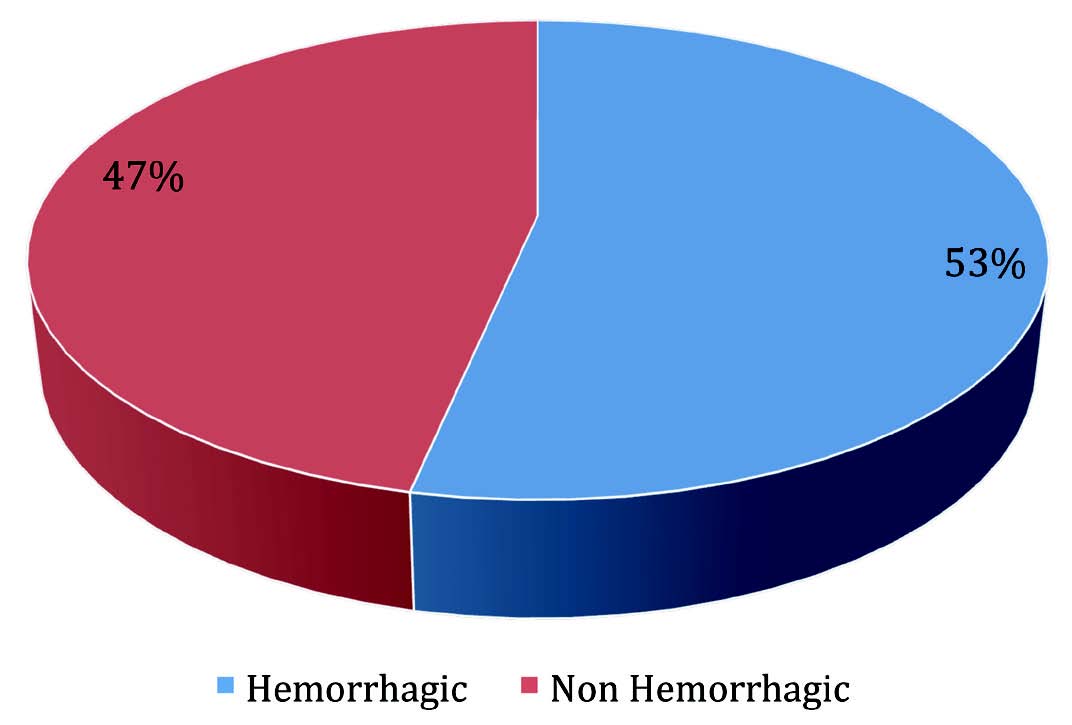
Figure 2: Color of fluid in metastatic adenocarcinoma effusions in cases studied.
In 23 (46%) cases with metastatic adenocarcinomas (MAC), adenocarcinoma cells were seen singly or as clusters with smooth contours. Acinar, papillary structures and proliferation spheres were also frequently seen. Nuclei were of variable sizes, with often-irregular contour, prominent nucleoli and high nucleus to cytoplasmic ratio. In six (12%) cases, it was not possible to convey a definitive diagnosis based on morphology alone and they were considered as suspicious for malignancy. These cases showed a presence of atypical cells with mild to moderate pleomorphism. The final diagnosis was based on clinical history, imaging studies, cytological features and histopathological examinations, which revealed 20 (40%) cases as benign effusions and 30 (60%) cases as malignant effusions.
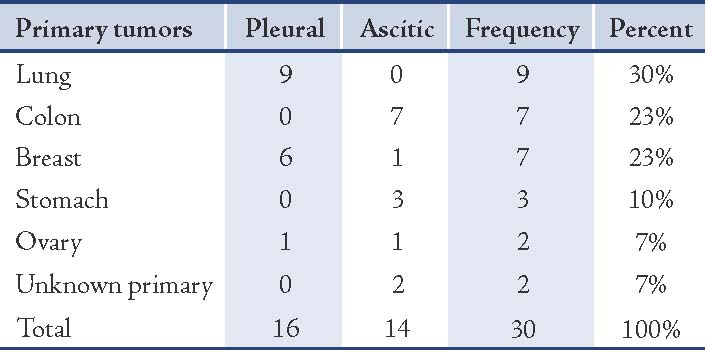
Of the 30 metastatic adenocarcinoma effusions, the diagnosis was confirmed by biopsy of the primary tumor in 28 cases, and by peritoneal biopsies in two cases. The 30 metastatic adenocarcinoma effusions were 16 pleural and 14 ascitic. Their primary sites are detailed in Table 1.
Table 1: The primary sites of metastatic adenocarcinoma encountered in effusions.
The benign effusions with reactive mesothelium were collected from eight (40%) cases of liver cirrhosis, five (25%) cases of congestive heart failure, four (20%) cases of pneumonia, two (10%) cases of nephrotic syndrome, and one (5%) case of pulmonary embolism.
In terms of sensitivity and specificity of cytological examination, suspicious cases were regarded as negative for malignancy for statistical purposes. When the cytological diagnoses were compared with the final clinicopathological diagnoses, there were seven false negative results and no false positive results. The encountered sensitivity of effusion cytology for detecting malignant cells was 77%, with 100% specificity, a positive predictive value of 100%, a negative predictive value of 74%, and an overall accuracy of 86%.
The false negative results were cases that had been diagnosed cytologically as reactive or suspicious; however, their final clinicopathological diagnoses were malignant with metastatic adenocarcinomas. Three suspicious effusions were the cause of the seven false negative cytology diagnoses which were finally diagnosed as malignant with metastasis from colonic and lung adenocarcinomas. Four reactive effusions also lead to false negative cytology diagnoses, but final diagnoses revealed them to be metastatic adenocarcinomas from breast, colon, lung, and stomach. The remaining three of the six suspicious cases were finally diagnosed as benign effusions, two cases from liver cirrhosis, and one case from pulmonary embolism.
All 50 effusion samples were subjected to immunocytochemical examination using two antibodies (anti-calretinin and anti-CEA). Immunocytochemical staining for calretinin was positive in 18/50 (36%) effusions. In all cases of benign effusions except two (18 out of 20 cases), reactive mesothelial cells had immunocytochemical positive staining with calretinin (CAL), in which they showed both cytoplasmic and nuclear staining. (Figs. 3 and 4)
Calretinin positivity was not noted in adenocarcinoma cells in any of 30 malignant cases. Mesothelial cells (if present) in the background of metastatic adenocarcinomas were positive for calretinin and were considered as internal controls. The sensitivity of CAL for detecting mesothelial cells was 90%, with 100% specificity, a positive predictive value of 100%, a negative predictive value of 94%, and 96% accuracy. (Table 2)
Table 2: Correlation between immunocytochemical staining results of CAL and final diagnosis in cases studied.
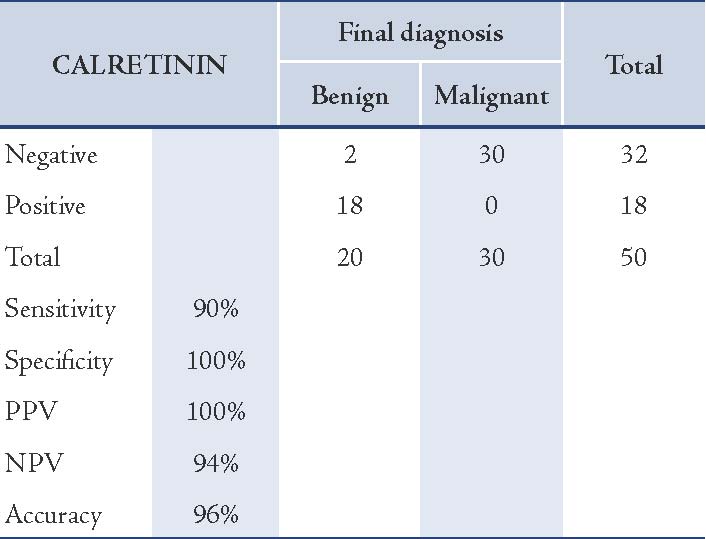
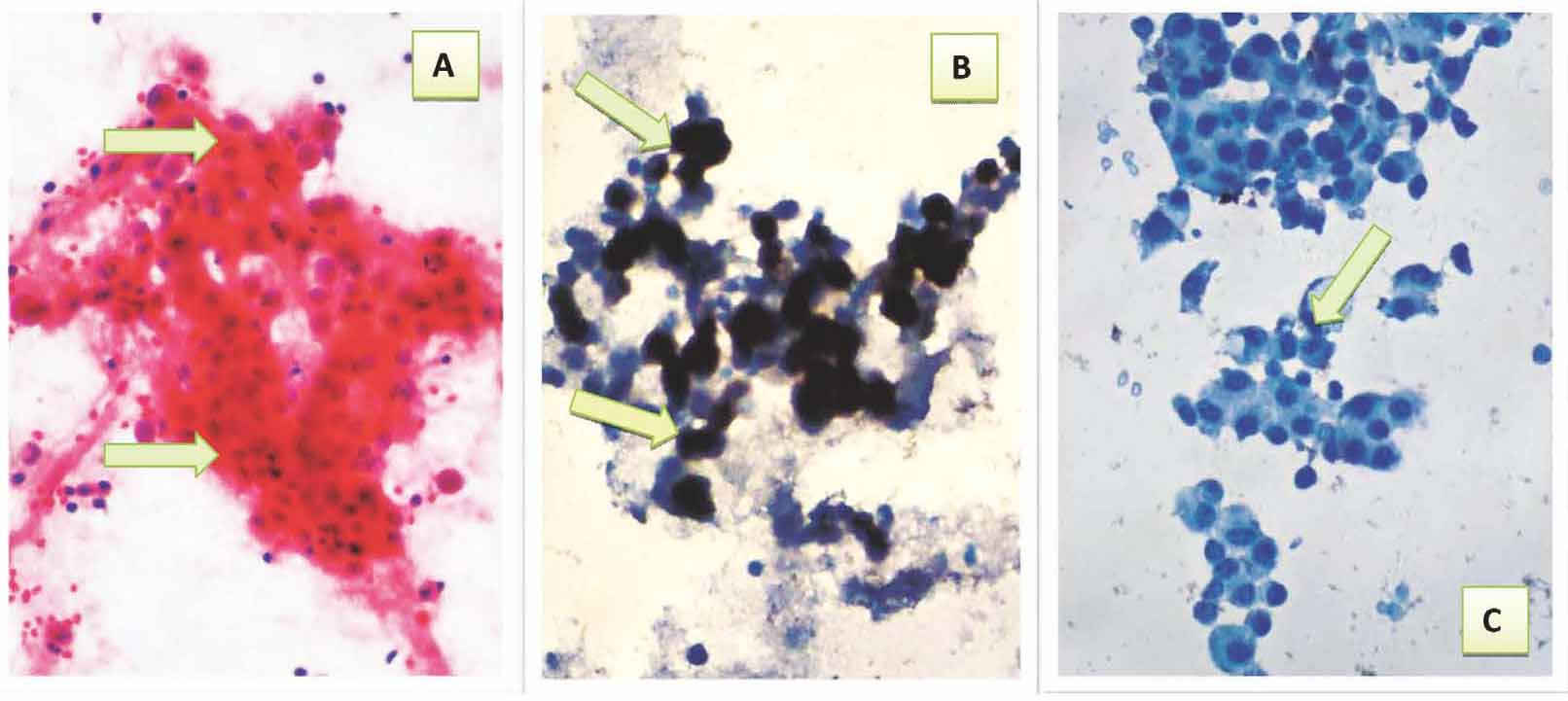
Figure 3: Reactive ascitic fluid showing: a- Cluster of benign looking reactive mesothelial cells with low nuclear to cytoplasmic ratio (arrows) (Pap40×). b- Positive immunocytochemical expression of CAL with brown cytoplasmic and nuclear staining with strong intensity (arrows) (40×). c- Reactive mesothelial cells with negative immunocytochemical expression of CEA (arrow) (40×).
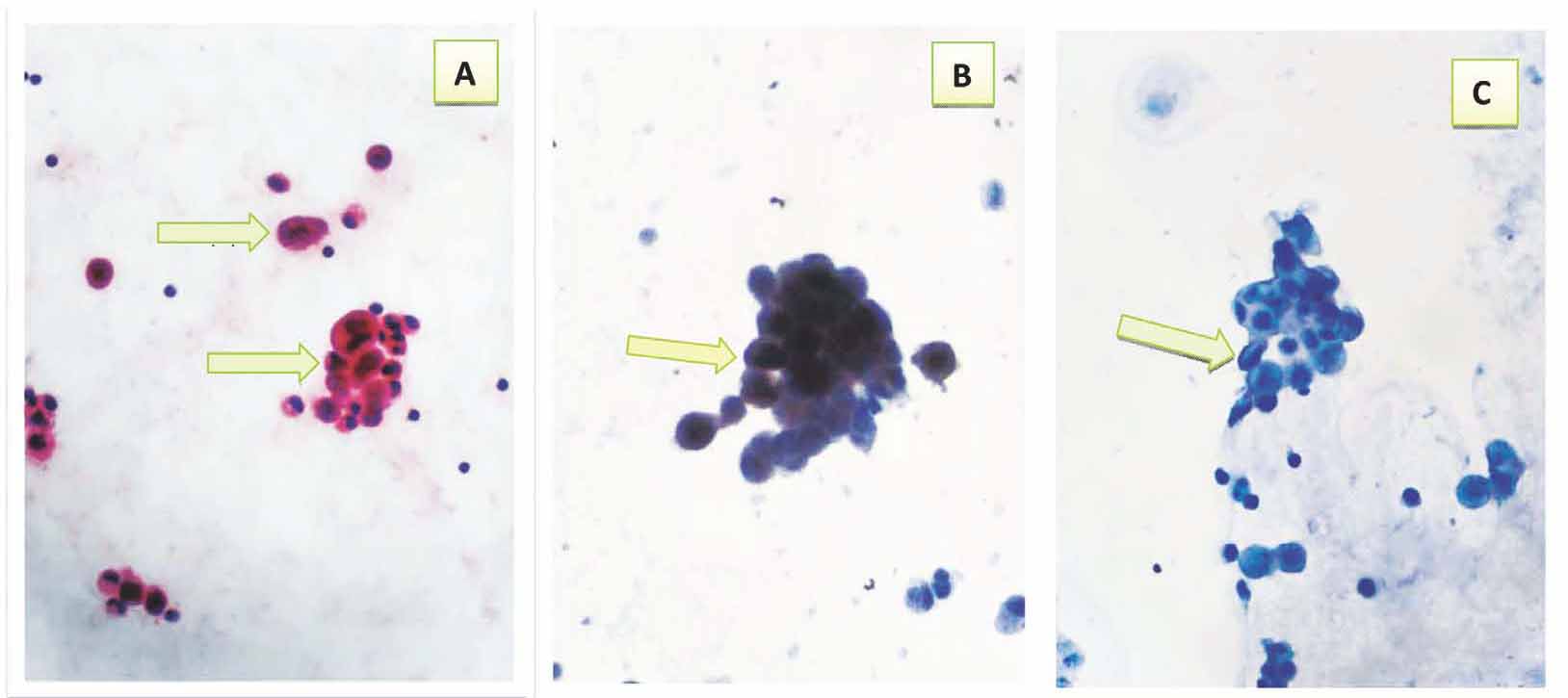
Figure 4: Suspicious ascitic fluid*showing: a- Cluster of few atypical cells with mild to moderate pleomorphism & relative high nuclear to cytoplasmic ratio (arrows) (Pap 40 ×). b- Positive immunocytochemical expression of CAL with brown cytoplasmic and nuclear staining with strong intensity (arrow) (40×). c- Negative immunocytochemical expression of CEA (arrow) (40×). * Final diagnosis was reactive effusion.
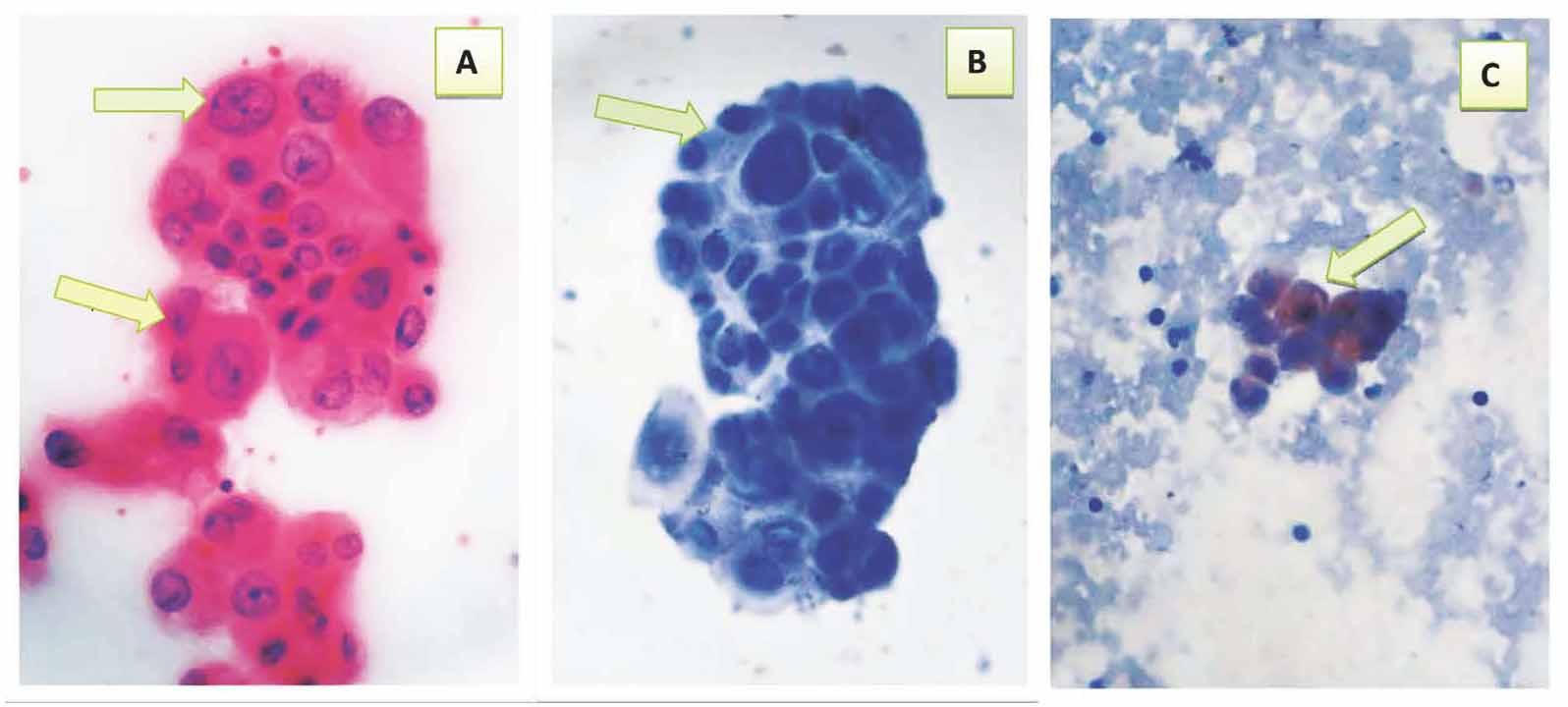
Figure 5: Suspicious ascitic fluid* showing: a- Cluster of atypical cells with moderate pleomorphism, some with high nuclear to cytoplasmic ratio & prominent nucleoli (arrows) (Pap 40×). b- Negative immunocytochemical expression of CAL (arrow) (40×). c- Positive immunocytochemical expression of CEA with brown cytoplasmic staining with moderate intensity (arrow) (40×). * Final diagnosis was Metastatic Adenocarcinoma effusion.
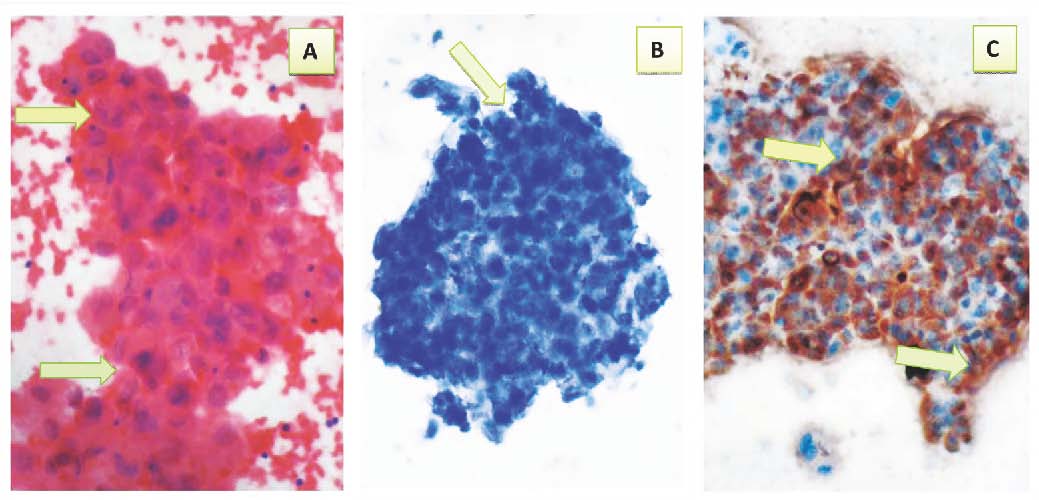
Figure 6: Malignant pleural fluid showing: a- Cluster of malignant epithelial cells with marked pleomorphism, high nuclear to cytoplasmic ratio, hyperchromasia and prominent nucleoli (arrows) (Pap 40 ×). b- Negative immunocytochemical expression of CAL (arrow) (40×). c- Positive immunocytochemical expression of CEA with brown cytoplasmic staining with strong intensity (arrows) (40X).
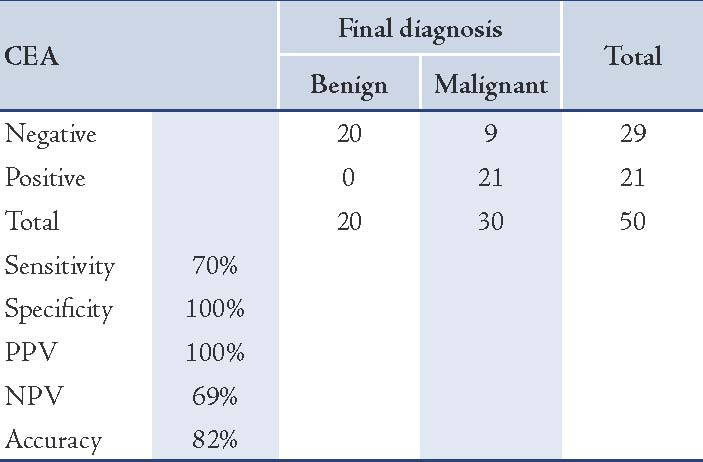
Immunocytochemical staining for CEA was positive in 21/50 (42%) effusions. CEA positive staining was obtained in adenocarcinoma cells in 21 of 30 cases with metastatic adenocarcinoma. Malignant cells showed cytoplasmic staining as shown in (Figs. 5 and 6). There were also nine false negative results. CEA immunocytochemical staining was absent in mesothelial cells, and none of the 20 benign effusions showed positive staining with CEA. The sensitivity of CEA for adenocarcinoma cells was 70%, with 100% specificity, a positive predictive value of 100%, a negative predictive value of 69%, and an accuracy of 82%. (Table 3)
Table 3: Correlation between immunocytochemical staining results of CEA and final diagnosis in cases studied.
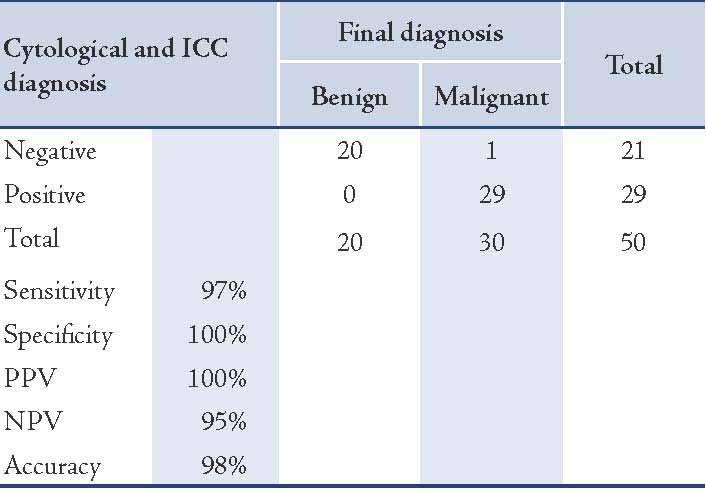
When both routine cytological examinations of effusion fluids were considered using Papanicolaou-stain and immunocytochemistry results in conjunction, an increase in sensitivity, specificity and accuracy of effusion diagnoses was observed. In addition, when a positive staining for CEA and a negative staining for CAL were considered as an indication of malignancy, the sensitivity of cytological and immunocytochemical results was shown to be 97%, with 100% specificity, a positive predictive value of 100%, a negative predictive value of 95%, and an overall accuracy of 98%. (Table 5)
Table 4: Distribution of CEA reactivity among the primary sites of metastatic adenocarcinomas.
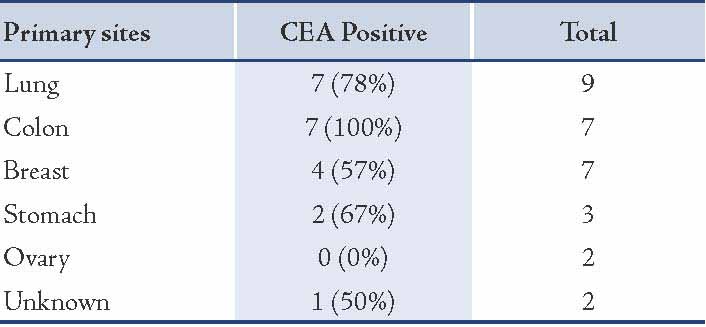
Table 5: Correlation between cytological, immunocytochemical results and final clinical diagnosis in cases studied.
When a positive staining for CAL as an indication of a benign etiology was considered, the combination of cytology and ICC results for CAL revealed no false negative results and the sensitivity of cytological and immunocytochemical results, specificity, positive predictive value, negative predictive value, and the overall accuracy were approximately 100%.
Discussion
The present study was conducted on 50 samples from patients who presented with serous effusions from which 36% were hemorrhagic. This observation was similar to that of other authors.11,12 Malignancy was the most common cause of hemorrhagic effusions which represented 89%, this is comparable with the findings of Ozcakar et al.12 Only 53% of malignant effusions were hemorrhagic in the current study, a finding which is in agreement with other reports.13
In this study, the most common primary site of malignancy with effusion was the lung, this has also been the case in other studies.14-17 In the current work, the sensitivity of effusion cytology for the detection of malignant cells was 77%, there was no false positive result, hence specificity was 100%, and with an overall accuracy of 86%. These findings are similar to those reported in another Iraqi study,18 as well as various other studies,19-21 with 53% and 61% sensitivity being reported for the detection of malignant cells respectively, and 100% specificity in both cases. Many factors predispose to diagnostic pitfalls in effusion fluid cytology, for instance; inappropriate collection, storage, processing of effusion specimens, multiple facets of reactive mesothelial cells, and paucity of tumor cells, as well as tumors which may produce effusions by blockage of lymphatics or blood vessels, or by causing inflammation of the serous membranes without invasion and thus cause tumor cells not to be identified in such effusions.1 The non-random selection of samples in the current study also increased both sensitivity and specificity of the tests. However, the limited number of cases was another factor that affected the results.
Immunocytochemistry provides a relatively simple, reliable and widely used technique to determine the origin of neoplastic tissue and investigate the behavior or progression of a given neoplasm.22-24 The preparation of cytological specimens to be examined immunocytochemically was the same procedure as that of conventional cytology. After smearing of the slides, they were quickly placed in the fixative to decrease the air-drying artifacts.
The method that was advocated in this study was the fixation of the smear in 95% ethyl alcohol as were fixed by Sato et al.25 for the concept of antigen retrieval. Heat-induced epitope retrieval technique was an additional important step for improving the quality of immunocytochemical staining of cytology smears in the current study. This method has also been advocated in many studies.25-28 A significant challenge associated with immunocytochemistry of effusions was finding and locating the cells of interest, since the same unique cells could not be present on more than one slide.
The present study has shown that staining for calretinin was positive in 18/50 (36%) effusion smears, these 18 were all cases of reactive effusions. Reactive effusions represented 20 out of the 50 studied cases. Adenocarcinoma cells did not show any reaction with calretinin in any of the 30 malignant cases. The sensitivity of CAL for detection of mesothelial cells was 90% with 100% specificity and 96% accuracy. Similar findings have been reported in other studies.2,5,15,16,20,26,29,30 The results of this study differ from those obtained by Lyons-Boudreaux et al.4 where the sensitivity of calretinin in identifying mesothelial cells was 67%. The differences in results are probably due to different antibody clones and sources, different immunocytochemical staining techniques and dilutions, and perhaps a variation in the evaluation of immunostaining results.
The current work revealed positive staining for CEA in 21/50 (42%) effusion smears. Adenocarcinoma cells with metastatic adenocarcinoma showed positive immunocytochemical staining for CEA in 21 out of 30 cases. In all effusion smears, mesothelial cells revealed negative staining for CEA. Overall, CEA exhibited 70% sensitivity, specificity 100% and 82% accuracy for adenocarcinoma cells. These observations are in accordance with other findings reported in the literature.10,16,30 However, findings from this study were in discordance with results obtained by Grefte et al.20 who reported 58% sensitivity for the detection of adenocarcinoma cells with CEA. Several factors could be attributed to this discordance such as the type antibody clone used, small sample size (only 34 cases selected by Grefte et al.),20 sample type (smears vs. cellblocks), procedural technique, and the range of tumor types used (high rate of positive CEA in gastrointestinal adenocarcinoma, while carcinoma of breast, lung, and ovary can express CEA in variable degrees). The adenocarcinoma of endometrium and prostate usually do not express CEA.
In the present study, CEA positive staining was demonstrated in high rates among effusions caused by metastatic adenocarcinomas of the colon 7/7 (100%), and 7/9 (78%) effusions caused by metastatic adenocarcinomas of lung. These finding are in agreement with other reports.16
The combined use of cytology examination and immunocytochemistry results was evaluated in the current study, and immunocytochemistry was observed to be a complementary technique to conventional cytology. Thus by combining the results of both techniques produced an increase in sensitivity and accuracy of diagnoses, particularly in malignant effusions.
In terms of diagnosis of malignancy, the combination of cytology and positive staining for CEA as well as negative staining for CAL reached a diagnosis in 29 of 30 metastatic adenocarcinoma cases, with 97% sensitivity, 100% specificity and 98% accuracy. Thus, these study findings confirm and expand on the results of previous studies about the use of these antibodies in a panel approach for the detection of adenocarcinoma cells in serous effusions.5,30
In the concept of benign etiology of effusions, the combination of cytological analysis and positive staining for CAL was found to exhibit 100% sensitivity, specificity and accuracy in patients with benign or reactive effusions. This suggests that a cell expressing positivity for CAL excludes the possibility of it being a carcinoma cell. Thus results of current work, as well as those of others, have shown that calretinin may be a reliable and specific marker for mesothelial cells in cytological preparations.2,5,15,26,30
Conclusion
Immunocytochemistry is a practical method which substantially improves the diagnostic accuracy of conventional cytology and Calretinin is a reliable and effective marker for the identification of mesothelial cells in effusions with a high sensitivity and specificity. Calretinin and CEA were found to be useful markers in the differentiation of reactive mesothelial cells from metastatic adenocarcinoma cells in cytological smears prepared from effusion fluids. Hence, the combination of both routine cytological examination and immunocytochemical staining for calretinin and CEA can greatly enhance the diagnostic accuracy of malignant effusions, particularly in equivocal cases.
Acknowledgments
We thank the staff at the Pathology Department, Medical College of Al-Nahrain University without exception for their assistance, and the staff at the cytology department of Al-Kadhmiya Teaching Hospital, as well as the staff from Central Public Health Lab. for their continuous support and assistance throughout the study. We also dedicate our extended appreciation to the staff at cytology department of Teaching Laboratories in Baghdad Medical City, Iraqi National Center and Specialized Surgical Hospital for their generous cooperation. No conflicts of interest to declare and no funding was received for this work.
References
1. Shidham VB, Falzon M. Serous effusions. In: Gray W, Kocjan G: editors. Diagnostic Cytopathology, 3rd Edition. Churchill Livingstone, Elsevier 2010; p 115-175.
2. He DN, Zhu HS, Zhang KH, Jin WJ, Zhu WM, Li N, et al. E-cadherin and calretinin as immunocytochemical markers to differentiate malignant from benign serous effusions. World J Gastroenterol 2004 Aug;10(16):2406-2408.
3. Butnor KJ. My approach to the diagnosis of mesothelial lesions. J Clin Pathol 2006 Jun;59(6):564-574.
4. Lyons-Boudreaux V, Mody DR, Zhai J, Coffey D. Cytologic malignancy versus benignancy: how useful are the "newer" markers in body fluid cytology? Arch Pathol Lab Med 2008 Jan;132(1):23-28.
5. Murugan P, Siddaraju N, Habeebullah S, Basu D. Immunohistochemical distinction between mesothelial and adenocarcinoma cells in serous effusions: a combination panel-based approach with a brief review of the literature. Indian J Pathol Microbiol 2009 Apr-Jun;52(2):175-181.
6. Davidson B. Ovarian carcinoma and serous effusions. Changing views regarding tumor progression and review of current literature. Anal Cell Pathol 2001;23(3-4):107-128.
7. Pereira TC, Saad RS, Liu Y, Silverman JF. The diagnosis of malignancy in effusion cytology: a pattern recognition approach. Adv Anat Pathol 2006 Jul;13(4):174-184.
8. Reid PT, Innes JA. Respiratory disease. In: Colledge NR, Walker BR, Ralston SH, editors. Davidson's Principles and Practice of Medicine, 21st Edition. Churchill Livingstone, Elsevier 2010; p 641-730.
9. Collier JD, Webster G. Liver and biliary tract disease. In: Colledge NR, Walker BR, Ralston SH, editors. Davidson's Principles and Practice of Medicine, 21st Edition. Churchill Livingstone, Elsevier 2010; p 919-984.
10. Agarwal C, Jain M. Utility of fibronectin in immuocytochemial differentiation of reactive mesothelial cells from metastatic malignant cells in serous effusions. Indian J Pathol Microbiol 2009 Jan-Mar;52(1):25-28.
11. Kushwaha R, Shashikala P, Hiremath S, Basavaraj HG. Cells in pleural fluid and their value in differential diagnosis. J Cytol 2008;25:138-143.
12. Ozcakar B, Martinez CH, Morice RC, Eapen GA, Ost D, Sarkiss MG, et al. Does pleural fluid appearance really matter? The relationship between fluid appearance and cytology, cell counts, and chemical laboratory measurements in pleural effusions of patients with cancer. J Cardiothorac Surg 2010;5:63.
13. Bibbo M, Wilbur DC, eds. Comprehensive Cytopathology, 3rd ed. Saunders, Elsevier 2008; p514- 577.
14. Hanley KZ, Facik MS, Bourne PA, Yang Q, Spaulding BO, Bonfiglio TA, et al. Utility of anti-L523S antibody in the diagnosis of benign and malignant serous effusions. Cancer 2008 Feb;114(1):49-56. Cancer Cytopathol.
15. Saleh HA, El-Fakharany M, Makki H, Kadhim A, Masood S. Differentiating reactive mesothelial cells from metastatic adenocarcinoma in serous effusions: the utility of immunocytochemical panel in the differential diagnosis. Diagn Cytopathol 2009 May;37(5):324-332.
16. Su XY, Li GD, Liu HB, Jiang LL. [Significance of combining detection of E-cadherin, carcinoembryonic antigen, and calretinin in cytological differential diagnosis of serous effusion]. Ai Zheng 2004 Oct;23(10):1185-1189.
17. Thapar M, Mishra RK, Sharma A, Goyal V, Goyal V. Critical analysis of cell block versus smear examination in effusions. J Cytol 2009 Apr;26(2):60-64.
18. Ali HH, Hammed AW. AL- Okabi ZT. Diagnostic value of CEA and CA19-9 levels in pleural effusions, correlation with cytology. Ir J Med Sci 2003;2(2):67-75.
19. Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002 Jun;346(25):1971-1977.
20. Grefte JM, de Wilde PC, Salet-van de Pol MR, Tomassen M, Raaymakers-van Geloof WL, Bulten J. Improved identification of malignant cells in serous effusions using a small, robust panel of antibodies on paraffin-embedded cell suspensions. Acta Cytol 2008 Jan-Feb;52(1):35-44.
21. Palaoro LA, Rocher AE, Rofrano J, Curi SM, Penzutti V. Utility of AgNOR, Immunocytochemistry for CEA and Tumer Markers for Diagnosis in Serous Fluids. Revista Brasileira de cancerologia 2008; 54:317-323.
22. Nussrat FL, Ali HH, Hussein HG, Al-Ukashi RJ. Immunohistochemical Expression of ki-67 and p53 in Colorectal Adenomas: A Clinicopathological Study. Oman Med J 2011 Jul;26(4):229-234.
23. Qasim BJ, Ali HH, Hussein AG. Immunohistochemical expression of estrogen and progesterone receptors in human colorectal adenoma and carcinoma using specified automated cellular image analysis system: a clinicopathological study. Oman Med J 2011 Sep;26(5):307-314.
24. Chaloob MK, Ali HH, Qasim BJ, Mohammed AS. Immunohistochemical Expression of Ki-67, PCNA and CD34 in Astrocytomas: A Clinicopathological Study. Oman Med J 2012 Sep;27(5):368-374.
25. Sato A, Torii I, Okamura Y, Yamamoto T, Nishigami T, Kataoka TR, et al. Immunocytochemistry of CD146 is useful to discriminate between malignant pleural mesothelioma and reactive mesothelium. Mod Pathol 2010 Nov;23(11):1458-1466.
26. Fetsch PA, Abati A. Immunocytochemistry in effusion cytology: a contemporary review. Cancer 2001 Oct;93(5):293-308.
27. Lozano MD, Panizo A, Toledo GR, Sola JJ, Pardo-Mindán J. Immunocytochemistry in the differential diagnosis of serous effusions: a comparative evaluation of eight monoclonal antibodies in Papanicolaou stained smears. Cancer 2001 Feb;93(1):68-72.
28. Politi E, Kandaraki C, Apostolopoulou C, Kyritsi T, Koutselini H. Immunocytochemical panel for distinguishing between carcinoma and reactive mesothelial cells in body cavity fluids. Diagn Cytopathol 2005 Mar;32(3):151-155.
29. Hongming L, Wang Xiaobin HC, et al. Application of Calretinin, VIM, ENTIN, CEA, EMA in Cytologic Diagnosis of Dropsy of Serous Cavity. Chinese Journal of Clinical Oncology 2001;3:19.
30. Ko EC, Jhala NC, Shultz JJ, Chhieng DC. Use of a panel of markers in the differential diagnosis of adenocarcinoma and reactive mesothelial cells in fluid cytology. Am J Clin Pathol 2001 Nov;116(5):709-715.
|