|
Abstract
Objective: This study aims to evaluate the diagnostic efficacy of adenosine deaminase in tubercular effusions.
Methods: This study was conducted at the Department of General Medicine and Cardiovascular and Thoracic Surgery, SKIMS, for a period of two years between November 2008 and November 2010. A total of 57 patients presenting with pleural effusions during the two-year study period, who presented with clinical manifestations suggestive of tuberculosis (i.e., the presence of productive cough, low-grade fever, night sweats, weight loss, and chest pain, especially if these symptoms last ≥4 weeks) were included in the study. If the patients presented with less than two of these symptoms, and especially if the clinical manifestations were of <4 weeks duration, they were excluded from the study.
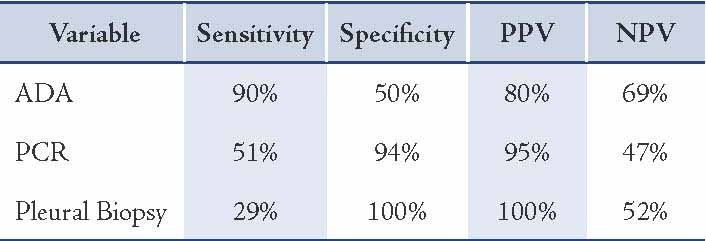
Results: The mean adenosine deaminase activity level in all the 57 patients was 109 U/L while the mean adenosine deaminase activity levels in pleural TB patients was 80 U/, and 64 U/L in the controls (p=0.381). Considering 40 U/L as the cut off, the results were positive in 35 out of 39 tuberculosis patients and 9 out of 18 controls. The sensitivity of adenosine deaminase for tubercular effusions worked out to be 90%, with only 50% specificity.
Conclusion: This study suggests that the estimation of adenosine deaminase activity in pleural fluid is a rapid diagnostic tool for differentiation of tubercular and non tubercular-effusions. The sensitivity and specificity of adenosine deaminase for tubercular effusions in this study was 90% and 50% respectively.
Keywords: Adenosine Deaminase (ADA); Pleural effusion; Acid fast bacillus staining (AFB staining).
Introduction
Tuberculosis (TB) still remains an important cause of morbidity and mortality worldwide. Recent estimates show that around 8-10 million new tuberculosis cases occur each year in the world and 2-3 million die.1 In developing countries, TB is one of the common opportunistic infections in people who are seropositive for human immunodeficiency virus (HIV).2 Tuberculosis is classified as pulmonary, extrapulmonary, or both.3 Pleural tuberculosis accounts for fewer than 1% of all exudative effusions in Western countries, occurring in only 3% to 5% of tuberculous patients and in developing countries like India, it is responsible for 30% to 80% of all pleural effusions encountered.4 Pleural effusions in TB usually have lymphocytic and exudative characteristics.5 Exudates are due to pleural inflammation (pleurisy), with an increased permeability of the pleural surface to proteinaceous fluid and various types of cells. Lymphatic obstruction may also contribute towards the accumulation of pleural fluid.6 Tubercular pleuritis is usually associated with primary disease and in those cases results from the rupture of subpleural focus, which may not be evident radiologically.7 It may also result from intra-pulmonary cavity or lymphohematogenous dissemination, or from an adjacent structure (e.g., lymph node or spine).7
Hypersensitivity to the tubercle bacillus also plays an important role in determining the occurrence and amount of pleural effusion. Tuberculous pleurisy is thought to be the result of a delayed hypersensitivity reaction in response to the presence of mycobacterial antigens in the pleural space.8 This immunologic reaction causes the stimulation and differentiation of lymphocytes, which release lymphokines, which in turn activate macrophages for an enhanced bactericidal effect.8 The difficulty in determining the cause of a pleural effusion, which is shown by the "unknown etiology" rates of up to 20% in some published case series, is largely due to the great variety of diseases that can bring about this condition. The etiology of pleural effusions depends on the geographical region, patient’s age, and advances in the diagnosis and treatment of the underlying cause.9
Due to the limitations of conventional tests and the delay of several weeks for mycobacterial culture results, there has been a resurgence of interest for newer rapid tests and biomarkers. New approaches for the rapid detection of mycobacterial growth have been developed with the aim to reduce the time needed for diagnosis. Measurement of adenosine deaminase (ADA) activity levels, have proven to be sensitive and specific for Pulmonary Tuberculosis (pTB) in special circumstances, such as in regions with a high prevalence of tuberculosis.10 The levels of ADA, an enzyme found in most cells, are increased in tuberculous pleural effusions, and this determination has acquired popularity as a diagnostic test in high-incidence areas for tubercular pleural effusion (TPE) because it is non-invasive, the assay is not expensive, and it is readily accessible. Polymerase chain reaction (PCR) is a new strategy used for tuberculosis diagnosis. PCR has been used to detect mycobacterial DNA in pleural fluid, with sensitivities ranging from 20% to 80% and specificities of 78% to 100%, depending on the area of the genome that is amplified and the technique used for DNA extraction.11 Pleural biopsy is usually considered important for the diagnosis of pleural effusions, especially for distinguishing between tuberculosis and neoplasia, even though tuberculous pleural fluid contains sensitive biochemical marker.12 Results of needle pleural biopsy have been reported to be positive in 50% to 80% of cases of tuberculous pleurisy.13
Demonstration of elevated pleural fluid ADA levels is useful in establishing the diagnosis of tuberculous effusions. ADA is an enzyme involved in purine catabolism. It is found in the majority of cells, but particularly in lymphocytes, where its concentration is inversely related to the degree of differentiation. An elevated pleural fluid ADA level predicts tuberculous pleuritis with a sensitivity of 90% to 100%.14 There are several isoforms of ADA, but the prominent ones are ADA1 and ADA2. ADA1 is present in all cells, whereas ADA2 is found only in monocytes.15 ADA2 is the predominant isoform in TPEs, accounting for most of the total ADA activity, in clinical practice; however, the difference in the use of total ADA or the isoform ADA2 is not considered to be significant. Furthermore, the isoenzyme assay is more expensive and not readily available.8 The reported cutoff value for ADA varies from 47 to 60 U/L.14 Specificity is increased when the lymphocyte/ neutrophil ratio in the pleural fluid (of >0.75) is considered together with an ADA concentration of >50 U/L.15
Theoretically, however, other lymphocyte-rich pleural effusions associated with live intracellular microorganisms could also have elevated ADA pleural fluid levels,16 including those caused by coccidioidomycosis and histoplasmosis. High levels of ADA have also been reported in non-infectious conditions associated with pleural fluid lymphocytosis, including malignant conditions (e.g., adenocarcinomas, leukemias, and lymphomas) and collagen vascular diseases (e.g., rheumatoid pleuritis and systemic lupus erythematosus), which makes the test less useful in countries with a low prevalence of tuberculosis.17,18 Thus, this study aims to determine the diagnostic efficacy of adenosine deaminase in tubercular effusions.
Methods
The patients included in this study were subjected to a thorough clinical examination and extensive investigations to establish a diagnosis of TB effusion. Complete hemogram, chest X-ray, chemistry tests including the Lactate dehydrogenase (LDH) and Mantoux test were done. Pleural fluid was subjected to a detailed analysis including total leukocyte count (TLC), differentiated leukocyte count (DLC), sugar, protein, LDH, gram stain, acid fast bacillus (AFB) stain, and M cells. Sputum AFB staining, pleural fluid ADA and PCR were done in all cases. Pleural biopsy was done in all patients after obtaining informed consent (patients who did not give consent/ or had any contraindication were not subjected to biopsy). Pleural biopsy was taken as a gold standard in this study.
Cases of pulmonary tuberculous (PTB) were defined as patients with clinical manifestations suggestive of tuberculosis presenting with one of the following: a) A positive Ziehl-Neelsen (ZN) results of the pleural fluid or other biological material (sputum, BAL, lymph node aspirate); b) Histopathologic findings suggestive of tuberculosis on pleural biopsy (presence of caseating granulomas was considered as tuberculosis); and c) Patients with clinical and radiologic findings that lack microbiological/histopathologic confirmation but responded positively to empirical therapy were also considered tuberculosis cases. A positive response to therapy was defined as the improvement of clinical and radiologic findings after 2 months of therapy.
The patients were divided into two broad categories; a) tuberculous (as per our broad definition of TB), and b) non-tuberculous. Labaratory evaluation of pleural fluid included a single specimen of pleural fluid (50-100 ml), which was submitted for cytological examination, ZN staining, ADA activity determination, and PCR. Pleural fluid samples were concentrated by centrifugation for 20 min at 10,000 rpm at 4ºC. The concentrated sample was taken for ZN staining. ADA activity was determined in 1 ml, of pleural sample using the calorimetric method described by Giusti and Galanti.19 A positive result was defined as a value >40 IU/L, which is based on previous studies of pleural fluid samples of patients with proven tuberculosis.
Results
A total of 57 patients with pleural effusion were enrolled in this study. The mean age of the study subjects was 42 ± 15 years with age ranging from 15-70 years. Out of the 57 patients, 42 (74%) were males and 15 (26%) were females. A majority of the males (n=17; 41%) were in their 4th and 5th decades of life and most of the females (n=11; 53%) were in their 3rd and 4th decades of life. The p value suggests that there was no significant age and gender bias in selecting the patients for this study. According to broad case definition of tuberculosis, the patients were broadly divided in two groups: tubercular (39/57; 68%) and non-tubercular (18/57; 32%). In the non-tubercular group, 11 (19%) patients had effusion of undetermined etiology, two (4%) patients had empyema and five (9%) patients were found to have malignant effusion.
Most of the patients diagnosed with pleural TB were in their 2nd, 3rd and 4th decades of life. Thirty-one out of 39 (79%) patients were below the age of 50 years, suggesting a higher incidence of tubercular effusions in the younger age group. A statistically significant association (p<0.009) was found between pleural TB and younger age. Productive cough and fever were the most common presenting symptoms of the patients with tuberculous effusion and both symptoms in combination were present in 69% of patients with pleural TB (27 of 39). Patients with pleural TB (22/39; 52%) presented with ≥2 symptoms. Moreover, 82% (32/39) of patients with pleural TB presented with productive cough compared with 61% (11/18) of patients with non-tuberculous effusions, (p=0.09; OR=2.9). Fever was most frequently present in patients with pleural TB (31/39; 80%) than in the non-tubercular group (10/18; 56%), [p=0.06; OR=3.1]. There p values for chest pain (p=0.001) and weight loss (p=0.001) were statistically significant among the two groups, with chest pain favoring non-tuberculous etiology and weight loss favoring tuberculosis.
Right-sided effusions were more commonly present in 64% (25/39) of patients with pleural TB than in the non-tubercular patients (p=0.549). The association was statistically insignificant, possibly because of the small sample size in this study. In this study, all patients with pleural tuberculosis had lymphocytic exudative effusions with mean lymphocyte differential count of 85 ± 10%. The pleural fluid total lymphocyte count of 100-1000 cells/mm3 was seen in 82% of patients of pTB. Elevated pleural fluid lactate dehydrogenase (LDH) of >400 U/ was seen in 87% of the patients with pTB. A significant association was observed between pleural TB and pleural fluid sugars <60 mg/dl (29/39; 74%; p=0.026) as well as with pleural fluid protein >5 g/dl (25/39; 64%; p=0.032).
Table 1: Etiology of pleural effusions in studied subjects.

ZN staining of pleural fluid was negative in all 39 patients with pleural tuberculosis. However, AFB was detected in seven patients on ZN staining from sputum (n = 4), BAL (n = 2), and lymph node aspirate (n=1). Mycobacterium tuberculosis was detected on AFB staining of lymph node aspirate from only one patient. PCR was positive in 20 out of the 39 diagnosed pleural tuberculosis cases, out of whom; two were also sputum smear positive for AFB. Seven patients were diagnosed based on findings of granulomatous inflammation on biopsy samples; six from pleural biopsy and one from the lymph node. Seven patients (not diagnosed by any confirmatory method) were considered as tuberculosis as per our broad case definition showing response to anti-tubercular therapy after two months. Thirty-five of the diagnosed tuberculosis patients had pleural fluid ADA levels above 40 U/L. All the 39 patients responded to anti-tubercular therapy.
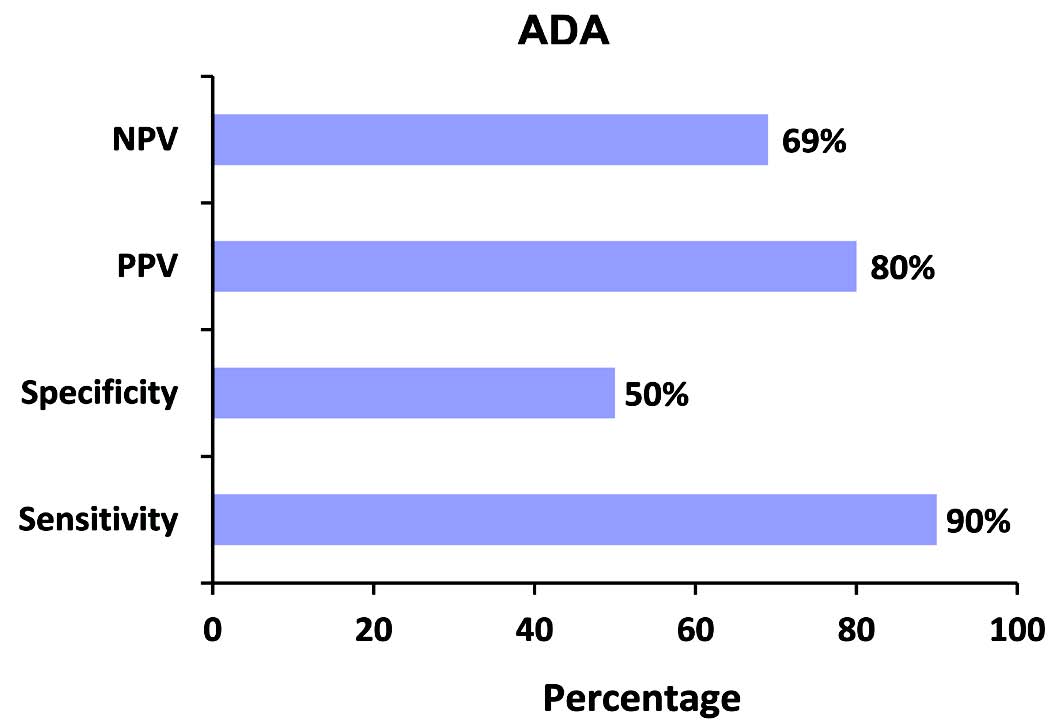
Figure 1: Negative predictive value (NPV), positive predictive value (PPV), specificity, and sensitivity of ADA in tuberculous effusions.
The mean ADA activity level in all the 57 patients was 109 U/L. The mean ADA activity levels in pleural TB patients was 80 U/ and 64 U/L in the controls (p=0.381). Considering 40 U/L as the cut off value, the results were positive in 35 out of 39 tuberculosis patients and in 9 out of 18 controls. The sensitivity of ADA for tubercular effusions worked out to be 90%, with a specificity of only 50%.
Pleural biopsy was performed in 37 out of the 57 studied patients. Of the 39 patients with pleural tuberculosis, pleural biopsy was performed in 21 patients. Histological findings suggestive of tuberculosis were found in six patients. In our, study the sensitivity of pleural biopsy was only 29% with 100% specificity.
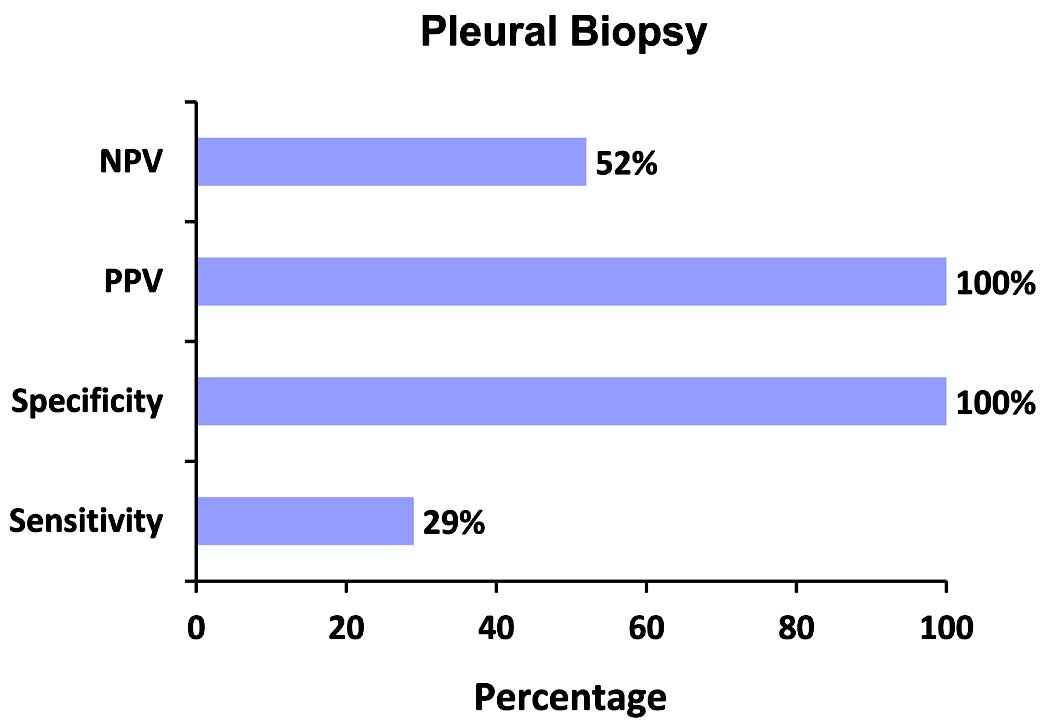
Figure 2: NPV, PPV, specificity and Sensitivity of pleural biopsy in tuberculous effusions.
Table 2: Comparison of sensitivity, specificity and predictive values of the diagnostic methods used in the study.
Discussion
This study was comprised of 57 cases of lymphocytic exudative pleural effusions, 39 diagnosed as tubercular and 18 as non-tubercular effusions. The average age of patients with tuberculous pleurisy is increasing and the disease is now commonly seen in the middle aged group. In the current study, 79% (31/39) of pTB patients were aged below 50 years, and 41% (16/39) were in the 20-40 years age group. The mean age of patients with tubercular pleurisy in our study was 37 years. The results were consistent with the results of Berger HW,20 where the mean age was 35 years, thus highlighting the increasing trend in age of tuberculous pleurisy patients.
Exudative lymphocytic pleural effusions commonly encountered in clinical practice often constitute difficult diagnostic problems. The two most common causes are malignancy and tuberculous effusions. For tuberculosis, the limitations of diagnostic tests include few positive staining and culture from pleural fluid, as well as time consumption for identification.
In present study, productive cough (82%) and fever (80%) were the most common presenting symptoms of patients with tuberculous effusions and both symptoms in combination were present in 69% of patients with pleural TB (27/39). Similar results were observed by Berger HW.20 Moudgil et al. also observed that cough was present in 70% of their cases.21 In this study, the pleural fluid white cell count was between 100-1000/mm3 in 82% of the patients. Thirty-four of the 39 patients had pleural fluid total leukocyte count below 2500. This was contrary to the findings of Berger HW, who reported a pleural fluid white blood cell count between 1,000 and 6,000/cu mm in 23 out of 41 patients (59%).20 Pleural fluid protein concentration of more than 5 gm/dl was seen in 64% and elevated lactic acid dehydrogenase (LDH) of more than 400 U/L was seen in 77% patients with pTB in our study. Similar results were reported by Berger HW,20 for both pleural fluid protein and LDH. Jadhav et al. found a glucose concentration less than 60 mg/100 mL in 52% of patients (13 out of 25), including seven with less than 30 mg/100 mL.22 They stated that a pleural glucose concentration of 30 mg/100 mL or less was diagnostic of tuberculosis and that the diagnosis of tuberculosis was unlikely if the value was above 60 mg/100 mL. The results of current study showed a pleural fluid glucose of less than 60 mg/dL in 74% of pTB patients and none had values less than 30 mg/dL. ZN staining of sputum had a sensitivity of 10% in this study and the results were consistent with the study conducted by Berger HW,20 where sputum smear positivity was 8%. Results of the present work were also in accordance with those reported by Chan CH.23
All the 39 patients with pTB received and responded to antitubercular treatment. Seven of these patients were considered as tubercular only on the basis of clinical response to anti-tubercular treatment (ATT) (as per our broad case definition). Out of the 39 patients with pleural tuberculosis, pleural biopsy was performed in 21 patients. Histological findings suggestive of tuberculosis were found in six patients. In present study, the sensitivity of pleural biopsy was only 29% with 100% specificity. The low sensitivity of pleural biopsy in this study is attributed to the fact that closed needle pleural biopsy was done in addition to the small sample size.
Considering 40 U/L as the cut off value, ADA was positive in 35 out of 39 tuberculosis patients and 9 out of 18 controls. The sensitivity of ADA for tubercular effusions worked out to be 90%, with only 50% specificity. Adenosine deaminase is considered an indicator of cell-mediated immunity and is found mainly in T lymphocytes and macrophages.24 Many studies have confirmed the utility of ADA for diagnosis of tuberculous pleural effusion. In a study done by Reechaipichitkul et al.25 the pTB diagnosis by ADA activity levels in the pleural fluid had a sensitivity of 80% and specificity of 80.5%. In 1999, Canbolat et al.26 showed the sensitivity of ADA assay to be 92% with 94.5% specificity, whereas, Ocana et al.27 reported the specificity of ADA to be 100% when pleural fluid ADA levels were above 70 U/L.
ADA activity measurements in present study also yielded good results in the diagnosis of pTB (sensitivity of 90%); however, ADA activity values may be increased due to other clinical entities. Valdes et al.12 have also reported high levels of ADA in patients with other causes of pleural effusions (mainly lymphomas, adenocarcinomas, systemic lupus erythematosus, and pneumonia). The best cutoff value was defined to be 40 U/L as was done by Danielle et al.14 in their study.
Conclusion
With the decline in the prevalence of TPE, the positive predictive value of pleural fluid ADA also declines, but the negative predictive value actually increases. Therefore, the measurement of pleural fluid ADA levels could be used to rule out a tuberculous etiology of lymphocytic pleural effusions, regardless of the rate of prevalence of TB.
Acknowledgements
The authors reported no conflicts of interest and no funding was received for this work.
References
1. Singh R, Singh RK, Tripathi AK, Gupta N, Kumar A, Singh AK, et al. Circadian periodicity of plasma lipid peroxides and anti-oxidant enzymes in pulmonary tuberculosis. Indian J Clin Biochem 2004 Jan;19(1):14-20.
2. Harries AD. Tuberculosis and human immunodeficiency virus infection in developing countries. Lancet 1990 Feb;335(8686):387-390.
3. Raviglione MC, O’Brien RJ. Tuberculosis. In. Fauci, Braunwald, Kasper, Hauser, Longo, Jameson, Loscalzo. Eds. Harrisons Principles of Internal Medicine, Tata McGraw Hill,17th ed. vol 1 p1010
4. Udwadia ZF, Sen T. Pleural tuberculosis: an update. Curr Opin Pulm Med 2010 Jul;16(4):399-406.
5. Aggarwal AN, Gupta D, Jindal SK. Diagnosis of tuberculous pleural effusion. Indian J Chest Dis Allied Sci 1999 Apr-Jun;41(2):89-100.
6. Sahn SA. The diagnostic value of pleural fluid analysis. Semin Respir Crit Care Med 1995;16(4):269-278 .
7. Rossman MD, Mayock RL. Pulmonary TB, in Schlossberg D, Ed. Tuberculosis. Springer verlag, New York, 1993; 95.
8. Kataria YP, Khurshid I. Adenosine deaminase in the diagnosis of tuberculous pleural effusion. Chest 2001 Aug;120(2):334-336.
9. Valdés L, Alvarez D, Valle JM, Pose A, San José E. The etiology of pleural effusions in an area with high incidence of tuberculosis. Chest 1996 Jan;109(1):158-162.
10. Villegas MV, Labrada LA, Saravia NG. Evaluation of polymerase chain reaction, adenosine deaminase, and interferon-gamma in pleural fluid for the differential diagnosis of pleural tuberculosis. Chest 2000 Nov;118(5):1355-1364.
11. de Wit D, Maartens G, Steyn L. A comparative study of the polymerase chain reaction and conventional procedures for the diagnosis of tuberculous pleural effusion. Tuber Lung Dis 1992 Oct;73(5):262-267.
12. Valdés L, Alvarez D, San José E, Juanatey JR, Pose A, Valle JM, et al. Value of adenosine deaminase in the diagnosis of tuberculous pleural effusions in young patients in a region of high prevalence of tuberculosis. Thorax 1995 Jun;50(6):600-603.
13. Lima DM, Colares JK, da Fonseca BA. Combined use of the polymerase chain reaction and detection of adenosine deaminase activity on pleural fluid improves the rate of diagnosis of pleural tuberculosis. Chest 2003 Sep;124(3):909-914.
14. Roth BJ. Searching for tuberculosis in the pleural space. Chest 1999 Jul;116(1):3-5.
15. Burgess LJ, Maritz FJ, Le Roux I, Taljaard JJ. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest 1996 Feb;109(2):414-419.
16. Dikensoy O, Namiduru M, Hocaoglu S, Ikidag B, Filiz A. Increased pleural fluid adenosine deaminase in brucellosis is difficult to differentiate from tuberculosis. Respiration 2002;69(6):556-559.
17. Maskell NA, Butland RJ; Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003 May;58(Suppl 2):ii8-ii17.
18. Lee YC, Light RW. Adenosine deaminase for lymphocytic pleural effusions. International Pleural Newsletter 2004;2:5-6.
19. Giusti G. Adenosine deaminase. Bergmeryer, HU eds. Methods of enzymatic analysis 1974; 1092-1099. Academic press. New York, NY.
20. Berger HW, Mejia E. Tuberculous pleurisy. Chest 1973 Jan;63(1):88-92.
21. Moudgil H, Sridhar G, Leitch AG. Reactivation disease: the commonest form of tuberculous pleural effusion in Edinburgh, 1980-1991. Respir Med 1994 Apr;88(4):301-304.
22. Jadhav AA, Bardapurkar JS. Diagnostic value of adenosine deaminase to differentiate exudates and transudates. Indian J Physiol Pharmacol 2007 Apr-Jun;51(2):170-174.
23. Chan CH, Arnold M, Chan CY, Mak TW, Hoheisel GB. Clinical and pathological features of tuberculous pleural effusion and its long-term consequences. Respiration 1991;58(3-4):171-175.
24. Jiménez Castro D, Díaz Nuevo G, Pérez-Rodríguez E, Light RW. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. Eur Respir J 2003 Feb;21(2):220-224.
25. Reechaipichitkul W, Kawamatawong T, Teerajetgul Y, Patjanasoontorn B. Diagnostic role of pleural fluid adenosine deaminase in tuberculous pleural effusion. Southeast Asian J Trop Med Public Health 2001 Jun;32(2):383-389.
26. Canbolat O, Ulusdoyuran S, Ozgen G, Ceyhan I, Gümüşlü F, Akbay A. The comparison of adenosine deaminase activity values with polymerase chain reaction results in patients with tuberculosis. J Clin Lab Anal 1999;13(5):209-212.
27. Ocana I, Martinez-Vazquez JM, Segura RM, et al. Adenosine deaminase in pleural fluids: test for diagnosis of tuberculous pleural effusions. Chest 1998;84:51-53.
|