|
Abstract
Objectives: Hypotestosteronemia has been reported in approximately half of type 2 diabetic men in general. This study aims to assess serum total testosterone levels in type 2 diabetics with erectile dysfunction and to correlate the degree of improvement between sildenafil citrate and testosterone levels.
Methods: A cross sectional and prospective comparative interventional study was conducted at the Diabetic Clinic of Assalam Teaching Hospital in Mosul, during the period from January 1, 2009 through to December 31, 2011. The study enrolled 120 type 2 diabetic males with erectile dysfunction who were analyzed with regard to age, duration of diabetes, duration and severity of erectile dysfunction, serum total testosteron levels and the degree of response to sildenafil citrate in terms of testosterone levels. The data were statistically analyzed using the independent two-sample Student t test, χ2 test and Pearson correlation test. A p-value of <0.05 was considered statistically significant.
Results: Thirty six percent of type 2 diabetic males with erectile dysfunction were found to have low serum testosterone levels. The hypotestosteronemic and normotestosteronemic subgroups were not significantly different in terms of mean age, duration of diabetes, reduction of libido, and reduction in erectile function. The rate and the degree of improvement of erection by sildenafil in the normo-and-hypotestosteronemic respondents were not significantly different, but the degree of improvement by sildenafil was significantly correlated to testosterone levels among the hypotestosteronemic group.
Conclusion: Hypotestosteronemia was found in 36% of type 2 diabetic males with erectile dysfunction. The degree of improvement of erectile dysfunction by sildenafil was directly proportional to the serum testosterone levels among the hypotestosteronemic group. Therapeutic supplement with testosterone preparation in the hypotestosteronemic diabetics with erectile dysfunction may improve their response to sildenafil.
Keywords: Type 2 diabetes; Sildenafil; Total testosterone level; Erectile dysfunction; Hypotestosteronemia, Low testosterone.
Introduction
Erectile dysfunction (ED) is a common problem in patients with chronic diseases. It is frequently observed in diabetic men with longstanding disease. The pathogenesis of ED is often multifactorial due to vascular, neurogenic, endocrine, pharmacological and psychological etiologies. Neurogenic nitric oxide (nNO) is still considered the most important modulator, among others, for the initiation of erection, whereas the endotheliogenic nitric oxide (eNO) seems essential for maintaining erection.1,2 Following sexual stimulation, nNO and eNO, are released in the corpora cavernosa of the penis to activate the enzyme guanylyl cyclase that leads to the transformation of guanosine monophosphate (GMP) into cyclic GMP (cGMP), which triggers both; relaxation of the helicine arteries and constriction of veins resulting in erection.3,4 Inhibition of phosphodiesterase-5 (PDE-5), the counteracting enzyme that degrades cGMP in the corpus cavernosum, provides the basis for the development of the drugs sildenafil, vardenafil and tadalafil that improve erection.3-5
ED is defined as the 'inability to achieve or maintain an erection adequate for sexual satisfaction.'6 The prevalence of ED in diabetic subjects ranges in different cross-sectional studies between 20% and 71%.7-9 ED in diabetes mellitus is multifactorial; hypotestosteronemia, autonomic neuropathy, and penile arterial insufficiency are all associated with a higher likelihood of ED in diabetes.10-12 Whereas, testosterone in humans is thought to play a larger role in sexual interest (libido) than in erectile capacity, testosterone deficiency in rats is associated with down regulation of the nNO, suggesting a trophic effect on erectile tissues.13,14 In addition to testosterone deficiency, diabetes in lab rats impairs eNO-mediated and nNO-mediated smooth muscle relaxation through the effects of cholesterol or advanced glycation end products on penile smooth muscle.15
Sildenafil citrate, the prototype of PDE-5 inhibitors, when administered before sexual activity increases the duration and rigidity of penile erection in response to visual sexual stimuli in a hospital setting,16 and enhances the ability to achieve erections and successful intercourse in a home setting.17 The drug was approved for use in erectile dysfunction by the US Food and Drug Administration (FDA) in 1998.18 PDE-5 inhibitors are only pharmacologically active when cGMP synthesis is activated after sexual arousal. The prescribing information for sildenafil recommends dosing 60 min before sexual activity.19 The first US trial concerning sildenafil use in men with type 1 and type 2 diabetes mellitus reported that 56% of the sildenafil-treated patients showed improvement in erectile function compared to 10% of those treated with placebo.20
Clinical trials in non-diabetic hypogonadal patients, in whom treatment with testosterone supplement alone failed, had shown that combined treatment with sildenafil and a testosterone preparation (T-gel) had a beneficial effect on ED.21 However, the response of diabetics with ED to sildenafil was not studied in correlation to native serum testosterone levels. The aim of this study is to assess serum total testosterone (TT) levels in a cohort of diabetic subjects with ED, and to correlate the degree of improvement by sildenafil citrate to TT levels.
Methods
This cross sectional and prospective comparative interventional study was conducted at the Diabetic Clinic of Assalam Teaching Hospital in Mosul, from January 1, 2009 through to December 31, 2011. The recruited subjects were 120 type 2 diabetics who were complaining of ED and/or depression in their sexual drive (libido). Their age ranged between 35-60 years and the mean age ± SD was 49.75 ± 7 years. The subjects who were excluded from the study included those aged above 60 years, smokers, uremic and subjects using drugs such as antiarrhythmic, diuretic, beta-blocker or antidepressants that may affect sexual function. The other exclusion criteria included subjects whose ED was of less than 3 months or whose ED was present before the diagnosis of diabetes.
The contributory data including age, duration of diabetes, co-morbidities, and drug history were recorded and tabulated. Sexual dysfunction was determined by presenting two direct questions to the enrolled subjects: "during your diabetes duration, have you got a decrement in your penile erection in comparison with your status before? When?", and "have you got any decrement in your sexual drive in comparison with before? When?" The responses to these two questions were assigned on a scale ranging from 0% to 100% as dictated by the patient himself. For example, the patient may record a 50% reduction of his power of erection and a 30% reduction in his libido in comparison with a previous time when he was quite satisfied years ago before the onset of diabetes.
The International Index for Erectile Function-5 (IIEF-5) that was used in such studies was abridged into two main criteria; the strength of erection and the sexual desire as these two criteria were the intended indices in the current study. Other markers of erectile dysfunction like noc tumescence (nocturnal spontaneous erection) or premature ejaculation were exempted.22 Serum TT levels were measured using enzyme-linked fluorescent immunoassay (ELFA) technique by Vidas instrument. The lowest normal TT level was regarded to be 10.4 nmol/L (3 ng/dl) and hypotestosteronemia was regarded to be present when the level was 10 nmol/L (2.9 nmol/L) or less.23
Thereafter, the subjects were informed about the effect of sildenafil. If its use was accepted by the subject and there were no contraindications, the subject's consent on using sildenafil as a trial was obtained. All the enrolled subjects were prescribed oral sildenafil citrate tablets in a fixed testing dose of 50 mg per day to be taken one hour before anticipated sexual intercourse. All the subjects were well informed about the possible side effects including headache, dyspepsia or sinus congestion. The subjects were allowed to use at least three doses of sildenafil citrate before recording their response. The subjects were instructed to take the drug apart from the main meals as food (fatty food in particular) is known to delay the absorption of sildenafil.24 The subjects' responses to sildenafil citrate were assessed in response to the question: "Did this drug improve your erections?" The degree of improvement was recorded in a percent grading.
All the data were edited and processed using the SSPS version 18. Comparison of continuous data (age, duration of diabetes and duration of ED) between normo- and hypotestosteronemics was performed using independent two sample Student t test. Comparison of the rate of response between normo and hypotestosteronemics was performed by the use of χ2 test. While the correlation of the degree of improvement by sildenafil to TT levels was performed using Pearson correlation. A p-value of <0.05 was considered statistically significant.
Results
Out of the 120 enrolled diabetics with ED, about one third (36%; n=44) were found to have hypotestosteronemia with a mean ± SD TT level of 7.6 ± 7.6 nmol/L. The remaining subjects (64%) were normotestosteronemic with a mean ± SD TT level of 16.5 ± 5.9 nmol/L (Table 1). The mean age of hypo- and normotestosteronemics was not significantly different (49.44 ± 6.35 vs. 50.79 ± 6.21 years, respectively; p=0.2) and neither was the mean duration of diabetes (7.29 ± 5.1 vs. 8.33 ± 4.87 years respectively; p=0.2). (Table 1)
In addition, the reductions in libido and erectile power among the hypo- and normotestosteronemics were not significantly different (29% ± 26% vs. 34.69% ± 29.79%; p=0.3), and (71.59 ± 20.16% vs. 69.21% ± 21.71%; p=0.5), respectively as shown in Table 1. In regard to sildenafil use by the hypotestosteronemics, 4 cases were exempted from using sildenafil (2 for their refusal and 2 for having clinical ischemic heart disease). Of the remaining 40 who used sildenafil, 32 (80%) improved variably and 8 (20%) had no improvement at all. The mean degree ± SD of improvement of power and amplitude of erection was 43.75% ± 30.75% (Table 2). In the normotestosteronimic group, 6 subjects were excluded from using sildenafil (3 for their refusal because of previous complications and 3 because of ischemic heart disease), 61 (87.1%) showed variable improvement with sildenafil and 9 (12.9%) showed no improvement at all. (Table 2)
The rate of respondents to sildenafil in the hypo-and- normotestosteronemics (80% vs. 87%, respectively) was not significantly different (p=0.3) and neither was the degree of improvement in erection power (43.75% ± 30.75% vs. 44.7% ± 27.31%; p=0.9) as shown in Table 2. However, the degree of improvement of erection by sildenafil in correlation to the levels of TT among the hypo- and normotestosteronemics starting from TT levels of ≤5.2 nmol/L up to ≤34.7 nmol/L, showed that the degree of improvement of erection by sildenafil was significantly correlated to the levels of TT among the hypotestosteronemics but not among the normotestosteronemics (r=0.28, p=0.05). (Figs. 1 and 2)
Table 1: Comparison between the normo and hypotestosteronemic type 2 diabetics with ED in terms of mean ± SD TT level, age, duration of diabetes, duration of ED, reduction of erection power and reduction of sexual desire.
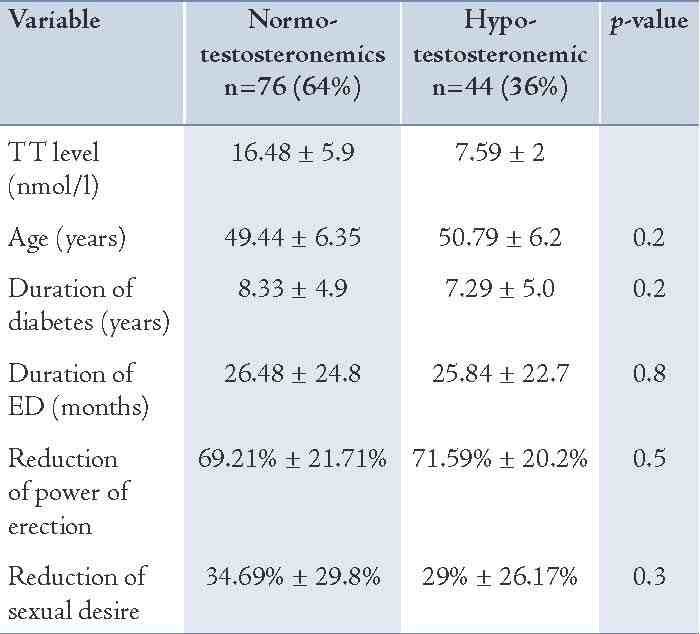
Table 2: The rate of respondents and the degree of improvement by sildenafil among the normo- and hypotestosteronemics.
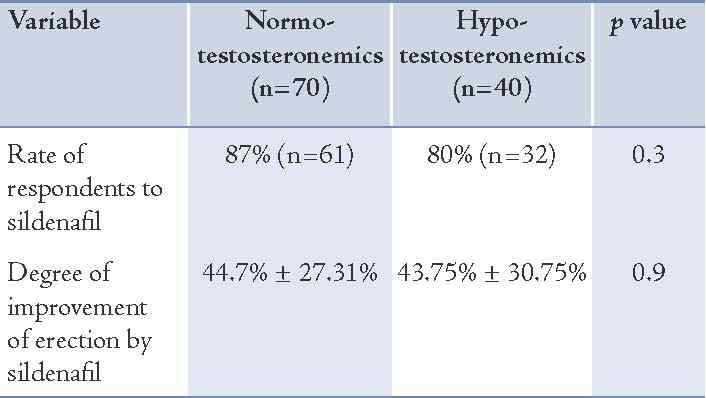
Figure 1: Correlation between the degree of improvement of erectile dysfunction by sildenafil and TT levels among normotestosteronemic type 2 diabetics with ED.
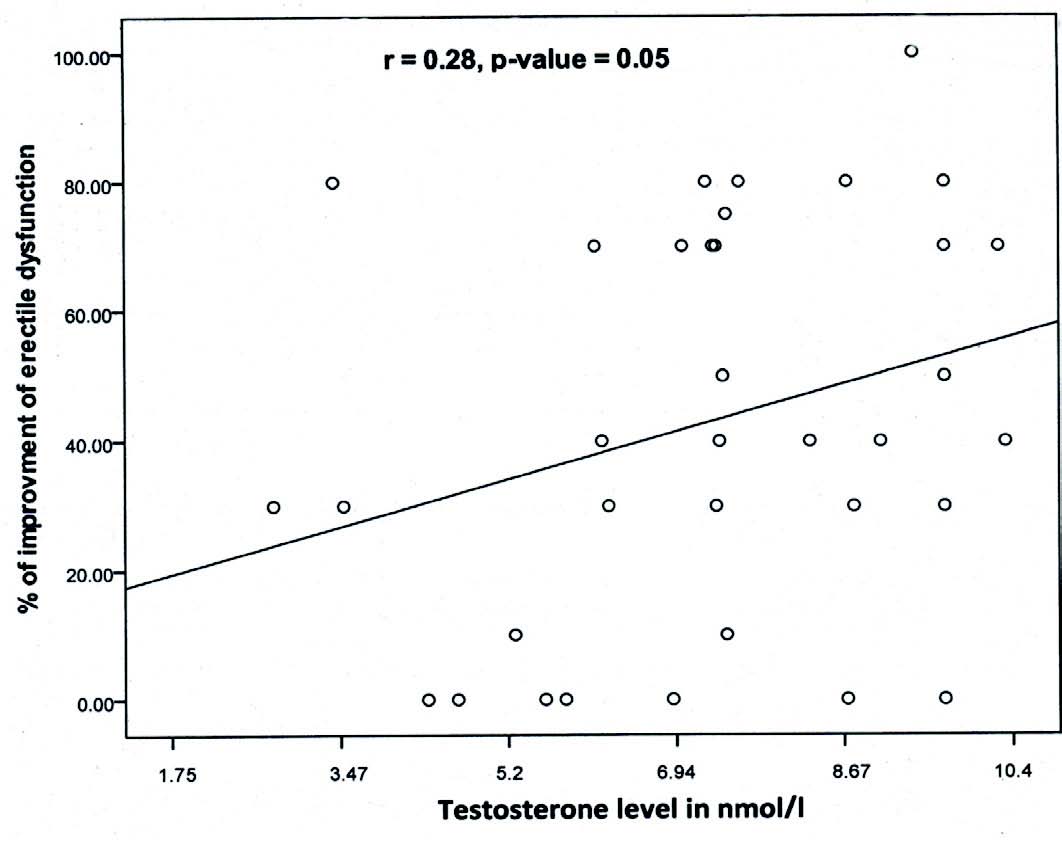
Figure 2: Correlation between the degree of improvement of erectile dysfunction by sildenafil and TT levels among hypotestosteronemic type 2 diabetics with ED.
Discussion
Hypotestosteronemia, sometimes referred to as hypoandrogenism or hypogonadism, is when the gonads (testes or ovaries) produce little or no hormones.25 Although Al-Kindi et al. had referred to calculated free testosterone or free androgen index as a better assessor of androgenic status in females than testosterone alone, in this study on diabetic males, testosterone was used to explore the androgenic status of the enrolled subjects.26 In males, hypogonadism is generally suspected when morning levels for TT are <10.4 nmol/L.23 Men with marked hypogonadism have a marked reduction in the frequency, amplitude and rigidity of erections.27,28 However, the level of hypogonadism required to induce change in sexual function is debatable.29-32 In regard to libido, Travision et al.33 reported that libido and testosterone displayed a significant association. However, the difference in testosterone levels between those with low libido and those without was small. Subjects with low libido showed an increased, but modest, probability of having low testosterone.34
In non-diabetic subjects, testosterone deficiency had been observed in about 6% to 12% of normal men over 45 years of age. Such deficiency reduces the quality of life and may pose important risks for sexual disorders, cardiovascular diseases, metabolic syndrome, type 2 diabetes and osteoporosis.35 In type 2 diabetes, testosterone deficiency affects up to 50% of men.36 Grossman et al. reported that 43% of men with type 2 diabetes have reduced TT levels and 57% have reduced free testosterone.37 In diabetic men with ED, aging, bad control, high total cholesterol, and anemia were all significantly correlated to decreased testosterone levels.38 The current study showed hypotestosteronemia in about one third of type 2 diabetics with ED. Reversing the trend, i.e., checking the prevalence of ED among the hypotestosteronemic diabetics, Ghazi et al. reported that 94.4% of the hypotestosteronemic diabetics had ED in comparison to 61% of eugonadal diabetics.31 Thus, only one third of diabetics with ED, as this study showed have hypotestosteronemia while most of the hypotestosteronemic diabetics have ED. In conformity with the results of the current work, Morales and Heaton reported that the rate of hypotestosteronemia among erectile dysfunction causes is low.39
The present study showed that the mean age of diabetics with ED was not significantly different between normo-and hypotestosteronemics. This finding partially deviates age from being a significant factor of hypotestosteronemia among diabetics with ED who were below 60 years (which was the age limit of the study population). The effect of diabetes rather than the age seems to be behind their hypotestosteronemia. Age and hypotestosteronemia appear not to always be correlated in subjects with ED. Morales and Heaton have reported that ED in men above 55 years of age does not imply the presence of hypogonadism, and if the two conditions; aging and hypogonadism are present, the indication for treatment of hypogonadism requires good clinical judgment.39
In the same way, the duration of diabetes that was shown to be statistically close in the hypo-and-normotosteronemic subgroups, implies that hypogonadism in diabetes is not a time dependent complication; it is rather a result of poor diabetic control as stated by Köhler et al.1 In spite of the concept of greater effect of testosterone deficiency on libido than on erection capacity, there were no significant differences in the degree of libido and erection regression in the hypo-and-normotestosteronemic diabetic subjects. Possible individual bias in over-scoring by the enrolled subjects of their sexual desire and their reluctance towards announcing loss of sexual desire in addition to loss of erection power might have rationalized the finding.
The introduction of PDE-5 inhibitors for the treatment of ED made a significant impact, both, on clinical efficacy and on increasing the awareness of the condition. Some men however, failed to respond to PDE-5 inhibitors alone. Interventional studies have demonstrated that testosterone replacement therapy in men with hypotestosteronemia improves erectile function in men who have previously failed to respond to PDE-5 inhibitors alone.21,40 Furthermore, it has been demonstrated that the full therapeutic potential of PDE-5 inhibitors will only become manifested in eugonadal state.41 The present study showed that the degree of improvement of erection by sildenafil is significantly correlated with TT levels among the hypotestosteronemic group. Such a correlation dictates that therapeutic optimization of serum testosterone levels of a diabetic subject with ED is a reasonable action to ameliorate his impotence.
Conclusion
Hypotestosteronemia was found in 36% of type 2 diabetic males with ED. The degree of improvement of erection power by sildenafil was directly proportional to serum TT levels among the hypotestosteronemics. Therapeutically raising TT levels of the hypotestosteronemic diabetics with ED may augment their response to sildenafil.
Acknowledgements
Thanks are due to Assalam Teaching Hospital in Mosul/Iraq particularly the staff of the Diabetic Clinic for their help in archiving the subjects' files, and to Dr Haitham Bader and Dr. Bassam Adwar for helping with the statistical analysis. The authors declare no conflict of interests. The research was fully funded by the researchers.
References
1. Köhler TS, Kim J, Feia K, Bodie J, Johnson N, Makhlouf A, et al. Prevalence of androgen deficiency in men with erectile dysfunction. Urology 2008 Apr;71(4):693-697.
2. Chitaley K, Webb RC, Mills TM. RhoA/Rho-kinase: a novel player in the regulation of penile erection. Int J Impot Res 2001 Apr;13(2):67-72.
3. Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res 2004 Jun;16(Suppl 1):S4-S7.
4. Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999 Mar;83(5A):21C-28C.
5. Chen J, Wollman Y, Chernichovsky T, Iaina A, Sofer M, Matzkin H. Effect of oral administration of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study. BJU Int 1999 Feb;83(3):269-273.
6. National Institutes of Health Consensus Statement, 1993.
7. Fedele D, Bortolotti A, Coscelli C, Santeusanio F, Chatenoud L, Colli E, et al. Erectile dysfunction in type 1 and type 2 diabetics in Italy. On behalf of Gruppo Italiano Studio Deficit Erettile nei Diabetici. Int J Epidemiol 2000 Jun;29(3):524-531.
8. Siu SC, Lo SK, Wong KW, Ip KM, Wong YS. Prevalence of and risk factors for erectile dysfunction in Hong Kong diabetic patients. Diabet Med 2001 Sep;18(9):732-738.
9. Penson DF, Wessells H. Erectile Dysfunction in Diabetic Patients. Diabetes Spectrum 2004;17(4):225-230 .
10. Schoeffling K, Federlin K, Ditschuneit H, Pfeiffer EF. Disorders of sexual function in male diabetics. Diabetes 1963 Nov-Dec;12:519-527.
11. Faerman I, Glocer L, Fox D, Jadzinsky MN, Rapaport M. Impotence and diabetes. Histological studies of the autonomic nervous fibers of the corpora cavernosa in impotent diabetic males. Diabetes 1974 Dec;23(12):971-976.
12. Bax G, Marin N, Piarulli F, Lamonica M, Bellio F, Fedele D. Rigiscan evaluation of specific nervous impairment in patients with diabetes and erectile disorders. Diabetes Care 1998 Jul;21(7):1159-1161.
13. Bancroft J, Wu FC. Changes in erectile responsiveness during androgen replacement therapy. Arch Sex Behav 1983 Feb;12(1):59-66.
14. Kwan M, Greenleaf WJ, Mann J, Crapo L, Davidson JM. The nature of androgen action on male sexuality: a combined laboratory-self-report study on hypogonadal men. J Clin Endocrinol Metab 1983 Sep;57(3):557-562.
15. Cartledge JJ, Eardley I, Morrison JF. Nitric oxide-mediated corpus cavernosal smooth muscle relaxation is impaired in ageing and diabetes. BJU Int 2001 Mar;87(4):394-401.
16. Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 1996 Jun;8(2):47-52.
17. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA; Sildenafil Study Group. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med 1998 May;338(20):1397-1404.
18. Kling J. From hypertension to angina to Viagra. Mod Drug Discov 1998;1:31-38.
19. Viagra (Sildenafil Citrate) Prescribing Information. 2006.
20. Rendell MS, Rajfer J, Wicker PA, Smith MD; Sildenafil Diabetes Study Group. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. JAMA 1999 Feb;281(5):421-426.
21. Greenstein A, Mabjeesh NJ, Sofer M, Kaver I, Matzkin H, Chen J. Does sildenafil combined with testosterone gel improve erectile dysfunction in hypogonadal men in whom testosterone supplement therapy alone failed? J Urol 2005 Feb;173(2):530-532.
22. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997 Jun;49(6):822-830.
23. A.D.A.M. Medical Encyclopedia. Last reviewed: October 14, 2010.
24. Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol 2002;53(Suppl 1):5S-12S.
25. Hirsch IB, Korenman SG, Stecher V, Diuguid C. Viagra" (sildenafil citrate): efficacy and safety in the treatment of erectile dysfunction in men with diabetes. American Diabetes Association Meeting, 19-22 June, 1999, San Diego, CA, USA.
26. Al Kindi MK, Al Essry FS, Al Essry FS, Mula-Abed WA. Validity of serum testosterone, free androgen index, and calculated free testosterone in women with suspected hyperandrogenism. Oman Med J 2012 Nov;27(6):471-474.
27. Tamler R, Deveney T. Hypogonadism, erectile dysfunction, and type 2 diabetes mellitus: what the clinician needs to know. Postgrad Med 2010 Nov;122(6):165-175.
28. Carani C, Zini D, Baldini A, Della Casa L, Ghizzani A, Marrama P. Testosterone and prolactin: behavioural and psychophysiological approaches in men. In: Bancroft J, editor. The Pharmacology of Sexual Function and Dysfunction. Amsterdam: Esteve Foundation Symposia, Vol 6. Excerpta Medica. Elsevier Science, 1995. pp 145–50
29. Rosen RC. Pharmacological effects on nocturnal penile tumescence. In: Bancroft J, editor. The Pharmacology of Sexual Function and Dysfunction. Amsterdam: Esteve Foundation Symposia, vol 6. Excerpta Medica. Elsevier Science; 1995. pp. 277–87.
30. Buena F, Swerdloff RS, Steiner BS, Lutchmansingh P, Peterson MA, Pandian MR, et al. Sexual function does not change when serum testosterone levels are pharmacologically varied within the normal male range. Fertil Steril 1993 May;59(5):1118-1123.
31. Ghazi S, Zohdy W, Elkhiat Y, Shamloul R. Serum testosterone levels in diabetic men with and without erectile dysfunction. Andrologia 2012 Dec;44(6):373-380.
32. Salmimies P, Kockott G, Pirke KM, Vogt HJ, Schill WB. Effects of testosterone replacement on sexual behavior in hypogonadal men. Arch Sex Behav 1982 Aug;11(4):345-353.
33. Travison TG, Morley JE, Araujo AB, O’Donnell AB, McKinlay JB. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab 2006 Jul;91(7):2509-2513.
34. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry 1984 Aug;145:146-151.
35. Anderson RA, Bancroft J, Wu FC. The effects of exogenous testosterone on sexuality and mood of normal men. J Clin Endocrinol Metab 1992 Dec;75(6):1503-1507.
36. Farrell JB, Deshmukh A, Baghaie AA. Low testosterone and the association with type 2 diabetes. Diabetes Educ 2008 Sep-Oct;34(5):799-806.
37. Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008 May;93(5):1834-1840.
38. Rabijewski M, Zgliczyński W. (Testosterone deficiency in elderly men). Pol Merkuriusz Lek 2009 Dec;27(162):517-523.
39. Morales A, Heaton JP. Hormonal erectile dysfunction. Evaluation and management. Urol Clin North Am 2001 May;28(2):279-288.
40. Aversa A, Isidori AM, Spera G, Lenzi A, Fabbri A. Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction. Clin Endocrinol (Oxf) 2003 May;58(5):632-638.
41. Gooren L. The role of testosterone in erectile function and dysfunction. J Mens Health Gend 2006;3(3):292-298 .
|