|
Abstract
Objectives: Stage III non-small cell lung cancer (NSCLC) has a poor prognosis. Reports suggest that five-year survival after current treatment is between 14 to 24 percent. The purpose of this retrospective study was to investigate the morbidity and mortality of patients diagnosed with stage III NSCLC and treated with pneumonectomy at the University of Kentucky Medical Center in Lexington, KY.
Methods: We reviewed the medical record and tumor registry follow-up data on 100 consecutive patients who underwent pneumonectomy for lung cancer at the University of Kentucky.
Results: We identified thirty-six patients in stage III who underwent pneumonectomy. Ten patients had surgery only, eight patients received adjuvant chemotherapy, and eighteen patients received neoadjuvant therapy. There was one surgical death in this series. Mean follow-up was 2.9 years. One-, three-, and five-year survival was 66%, 38%, and 38%, respectively. Five-year survival for the group with adjuvant therapy was 60%.
Conclusion: Most lung cancer patients present with advanced disease and the prognosis remains poor. Our experience indicates resection offers an above average chance of long-term survival when supplemented with neoadjuvant and/or adjuvant therapy.
Keywords: Pneumonectomy; Non-small cell lung cancer; Resection; Lobectomy; Surgery; Chemotherapy; Neoadjuvant therapy; Survival.
Introduction
Lung cancer is the leading cause of cancer deaths among men and women in the United States with an estimated 156,940 deaths attributed to the disease in 2011.1 Pneumonectomy is the surgical removal of an entire lung and is one of the surgical treatment options available for lung cancer, along with lobectomy and wedge resection. The majority of lung cancer resections involve lobectomy, with pneumonectomy being a relatively uncommon procedure representing less than 15% of all lung cancer resections.2 Along with surgery, radiation and chemotherapy also play a vital role in the treatment of lung cancer. For patients diagnosed with non-small cell lung cancer (NSCLC), the treatment involves a combination of surgery, chemotherapy, and radiation. However, for patients diagnosed with small cell lung cancer, surgery is not part of the treatment regimen.
The purpose of this retrospective study was to investigate the morbidity and mortality of patients diagnosed with stage III NSCLC and treated with pneumonectomy at the University of Kentucky Medical Center in Lexington, KY.
Methods
Between September 1998 and September 2009, one hundred patients underwent pneumonectomy for primary lung cancer at the University of Kentucky Medical Center in Lexington, Kentucky. The data for the study were obtained by analyzing the patient records, which included electronic records as well as paper charts. This study was reviewed and approved by the University of Kentucky Institutional Review Board (approval #08-0529-P2H). Patient consent was deemed unnecessary due to patient’s non-identifying data collected for study. The demographic data collected included age, sex, histological type, and pathologic stage of lung cancer, pneumonectomy site, and adjuvant and/or neoadjuvant therapy status (Table 1). Additionally, the postoperative complications and operative mortality data were obtained from the operative reports and hospital stay records. The postoperative complications included all complications during the postoperative hospital stay, while the postoperative mortality included any mortality occurring within 30 days post-pneumonectomy or any mortalities occurring during the same hospitalization. Long-term follow-up and survival data were obtained using the Kentucky Cancer Registry. Mediastinoscopy was used to stage the patients in this study. We utilized American Joint Committee on Cancer (AJCC) seventh edition TNM staging criteria in this study. Additionally, we defined overall survival as the percentage of subjects that survived at the end of a defined period, which in our case was 1 year, 3 year or 5 years.
Five-year survival was analyzed using the Kaplan-Meier method. Survival was compared across the side of resection and the level of therapy received (adjuvant and/or neo-adjuvant therapy) using log-rank tests. Statistical significance was set at p < .05. SPSS™ version 19 statistical software was used for all analyses (IBM Corp., New York, NY).
A total of one hundred patients were evaluated for this study from which thirty-six patients were identified with stage III disease. Of the total 36 subjects in this study, 5 subjects had N2 disease (14%). Median age of the patients was 61 years with a range of 30-79 years. There were twenty-three male and thirteen female patients. Twenty patients underwent left pneumonectomy (56%), and sixteen patients underwent right pneumonectomy. Squamous cell carcinoma was identified in twenty-three patients, adenocarcinoma in five patients, undifferentiated non-small cell carcinoma in seven patients, and mixed (adenosquamous) in one patient. Eighteen patients received neoadjuvant chemotherapy and/or radiation, with four of these patients eventually receiving additional therapy after pneumonectomy; eight patients received adjuvant chemotherapy and/or radiation, and ten patients received surgery alone (Table 1).
Results
Major postoperative complications occurred in 16 patients, resulting in a complication rate of 44%. The most common complication was atrial fibrillation occurring in six patients, with urinary tract infection being the second most common and occurring in three patients (Table 2). There was one postoperative death, resulting in a 3% postoperative mortality rate. The cause of death was acute myocardial infarct. There were no intraoperative deaths.
Mean follow-up was 2.9 years. Median survival was 23.9 months (95% C. I. 10.1-37.8 months). Overall, one-, three-, and five-year survival was 66%, 38%, and 38%, respectively (Figure 1). Five-year survival for the group with adjuvant therapy was 60% compared to 33% for the group that received neoadjuvant therapy, and 30% for the group that received surgery only (Figure 2). The one-, three-, and five-year survival for patients who underwent right pneumonectomy was 56%, 29%, and 29%, respectively, compared to 74%, 45%, and 45% for left pneumonectomy. The difference in survival between left and right pneumonectomy is not statistically significant (p-value = 0.343).
Table 1: Clinical characteristics of patients with stage III cancer; n=36.
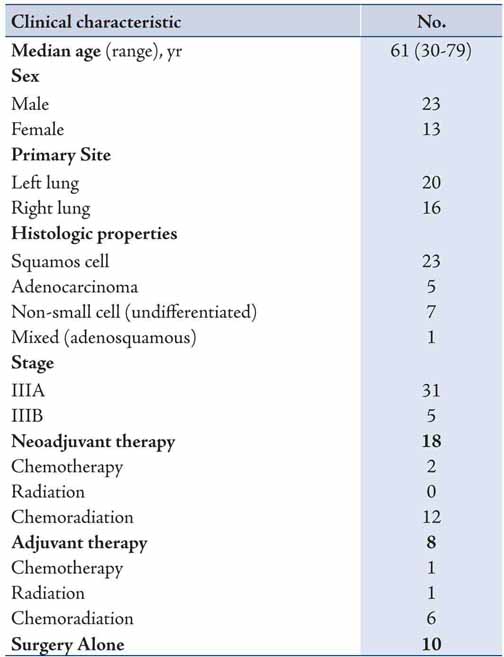
Table 2: Postoperative complications in patients with stage III NSCLC.
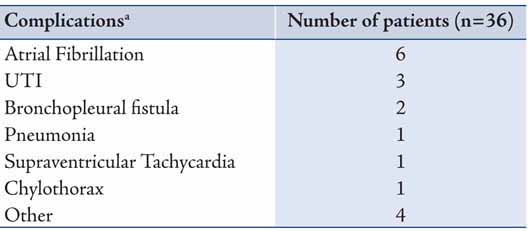
Figure 1: Survival function after pneumonectomy in stage III non-small cell lung cancer. Stage 3 patients, n=36. 1y survival = 66%, 3y survival = 38%, and 5y survival = 38%.
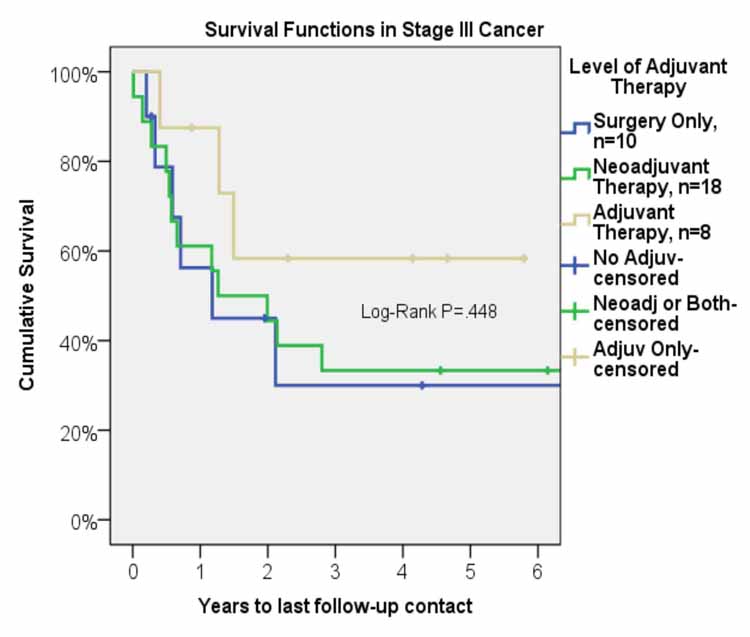
Figure 2: Survival function after pneumonectomy in stage III non-small cell lung cancer by level of adjuvant therapy.
Discussion
The overall 1-, 3-, and 5-year survival for patients in this report compares favorably to the data in recently published literature. In the study by Ferguson, et al. the overall 1-, 3-, and 5-year survival was 52%, 28%, and 21%, respectively, for stage III NSCLC, while Friedel et al. noted 5-year overall survival of 22%.3,4 Stamatis et al. report 5-year overall survival of 36% for stage IIIA disease and 26% for stage IIIB disease.5 Additionally, Decaluwe et al. report a 5-year survival of 33%.6 Results published in recent literature are comparable to the results we observed in our study, with favorable 5-year overall survival of 38% for stage III NSCLC.
Many studies have tried to understand the role of neoadjuvant therapy in the treatment of patients diagnosed with NSCLC. The 30-day postoperative mortality for our neoadjuvant group was 5.6% with 1 death out of 18 patients. The literature shows mixed results with some publications indicating that neoadjuvant therapy results in a significant increase in postoperative mortality. A study published by Albain et al. showed a high postoperative mortality rate of 26% (14/54) for stage III NSCLC patients who received neoadjuvant chemoradiation.7 Daly et al. report a postoperative mortality rate of 13.3% (4/30) and concluded that neoadjuvant chemoradiation therapy is associated with high mortality but results in significant long-term survival for patients who survive with a 5-year survival rate of 38%.8 Similarly, Venuta et al. observed a mortality rate of 12.5% (4/32) for patients who received neoadjuvant therapy and concluded that pneumonectomy should only be performed in carefully selected patients.9 However, Alan et al. report a low postoperative mortality rate of 5.6% and conclude that there is no significant increase in postoperative mortality after pneumonectomy in those who received neoadjuvant chemotherapy.10 Similarly, Ng et al. did not observe any significant difference in postoperative mortality between the group that underwent neoadjuvant therapy (with no mortalities out of 17 patients) and the group that did not receive neoadjuvant therapy.11 In our study, the postoperative mortality of 5.6% for patients with stage III disease undergoing neoadjuvant therapy was relatively low, and there was not a significant increase when compared to the overall mortality rate of 3%. With the results we obtained at our institution, we can conclude that induction therapy can be safely performed for patients with stage III disease undergoing pneumonectomy.
For patients who underwent neoadjuvant therapy, the 1-, 3-, and 5-year survival was 60%, 33%, and 33%. For patients who only received surgical intervention, the 1-, 3, and 5-year survival was 56%, 30%, and 30%. The long-term survival between these two groups was comparable and the differences are not statistically significant. Weder et al. report 3-year survival of 43% and 5-year survival of 38%.12 Allen et al. report similar survival rates with 1-, 3-, 5-year survival of 70%, 49%, and 20%, respectively, for patients with stage III disease who underwent neoadjuvant chemoradiation followed by pneumonectomy.13 Similarly, Paul et al. report 5-year survival of 22% for patients with stage IIIA disease who underwent induction therapy prior to pneumonectomy.14 Additionally, a phase III randomized control trial by Albain et al. showed 5-year survival of 25% for patients who received radiotherapy plus chemotherapy prior to undergoing pneumonectomy.15 The 5-year survival of 33% that we observed at our institution is comparable to the results published in recent literature and allows us to conclude that neoadjuvant therapy is a valuable option for treatment of patients diagnosed with stage III NSCLC as it converts many non-resectable tumors to a resectable stage and offers long-term survival benefits. Neoadjuvant therapy should be considered as part of overall therapy for stage III NSCLC.
In our study, a 5-year survival of 60% for the adjuvant therapy group was significantly higher than the group that received neoadjuvant therapy and was also higher than the group that received surgery alone, 33% and 30%, respectively. The International Adjuvant Lung Cancer Trial from 2004 looked at the role of adjuvant chemotherapy. The study divided patients with stage IB to IIIA disease (39% of patients had stage IIIA disease) into two groups, with one group receiving adjuvant chemotherapy and the other group receiving surgery alone. In addition, patients with stage IIIA disease also received adjuvant radiation therapy regardless of which group they were assigned to. The adjuvant chemotherapy group showed improved survival (5-year survival of 44.5% versus 40% for control group, p < 0.03) with stage IIIA patients showing the greatest benefit. Additionally, the disease-free survival was significantly higher in the chemotherapy group (5-year survival of 39.4% vs. 34.3%, p < 0.003.)16 In addition, a recently published study by Douillard et al. showed significantly improved 5-year survival (42% versus 26%, p=0.013) for stage IIIA patients who received adjuvant chemotherapy.17 With these results, the American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (2nd Edition) gave a 1A recommendation to the use of adjuvant chemotherapy in patients found to have incidental IIIA N2 disease. The ACCP recommendation for adjuvant radiotherapy is 2C, with the comment that adjuvant radiotherapy should be considered after adjuvant chemotherapy to reduce local recurrence.18 Because the most important factor that results in reduced survival in patients with lung cancer is systemic recurrence, adjuvant therapy, especially chemotherapy, may play an important role in improving the long-term survival by reducing systemic spread.
Conclusions
In our study, a 60% 5-year survival rate for the adjuvant therapy group demonstrates the benefit of adjuvant therapy in increasing the long-term survival in patients with stage III disease. Results from our study provide further evidence for a definitive role of adjuvant therapy in treatment of stage III disease to improve long-term survival.
In summary, for patients diagnosed with stage III NSCLC, multimodality therapy may offer the best survival benefits. Neoadjuvant therapy prior to pneumonectomy converts many non-resectable tumors to a resectable stage and subsequently offers long-term survival benefits, and in our experience, it did not significantly increase the postoperative mortality. Adjuvant therapy may significantly improve long-term survival and should be considered an important part of treatment for patients diagnosed with stage III NSCLC. Most lung cancer patients present with advanced disease and the prognosis remains poor. In the surgeon’s hands, resection offers an above average chance of long-term survival when supplemented with neoadjuvant and/or adjuvant therapy.
References
1. Lung cancer (non-small cell). From http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics Accessed March 2013.
2. Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest 1992 May;101(5):1332-1337.
3. Ferguson MK, Karrison T. Does pneumonectomy for lung cancer adversely influence long-term survival? J Thorac Cardiovasc Surg 2000 Mar;119(3):440-448.
4. Friedel G, Budach W, Dippon J, Spengler W, Eschmann SM, Pfannenberg C, et al. Phase II trial of a trimodality regimen for stage III non-small-cell lung cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol 2010 Feb;28(6):942-948.
5. Stamatis G, Eberhard W, Pöttgen C. Surgery after multimodality treatment for non-small-cell lung cancer. Lung Cancer 2004 Aug;45(Suppl 2):S107-S112.
6. Decaluwé H, De Leyn P, Vansteenkiste J, Dooms C, Van Raemdonck D, Nafteux P, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009 Sep;36(3):433-439.
7. Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT III, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995 Aug;13(8):1880-1892.
8. Daly BD, Fernando HC, Ketchedjian A, Dipetrillo TA, Kachnic LA, Morelli DM, et al. Pneumonectomy after high-dose radiation and concurrent chemotherapy for nonsmall cell lung cancer. Ann Thorac Surg 2006 Jul;82(1):227-231.
9. Venuta F, Anile M, Diso D, Ibrahim M, De Giacomo T, Rolla M, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 2007 Apr;31(4):714-717.
10. Alan S, Jan S, Tomas H, Robert L, Jan S, Pavel P. Does chemotherapy increase morbidity and mortality after pneumonectomy? J Surg Oncol 2009 Jan;99(1):38-41.
11. Ng T, Birnbaum AE, Fontaine JP, Berz D, Safran HP, Dipetrillo TA. Pneumonectomy after neoadjuvant chemotherapy and radiation for advanced-stage lung cancer. Ann Surg Oncol 2010 Feb;17(2):476-482.
12. Weder W, Collaud S, Eberhardt WE, Hillinger S, Welter S, Stahel R, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010 Jun;139(6):1424-1430.
13. Allen AM, Mentzer SJ, Yeap BY, Soto R, Baldini EH, Rabin MS, et al. Pneumonectomy after chemoradiation: the Dana-Farber Cancer Institute/Brigham and Women’s Hospital experience. Cancer 2008 Mar;112(5):1106-1113.
14. Paul S, Mirza F, Port JL, Lee PC, Stiles BM, Kansler AL, et al. Survival of patients with clinical stage IIIA non-small cell lung cancer after induction therapy: age, mediastinal downstaging, and extent of pulmonary resection as independent predictors. J Thorac Cardiovasc Surg 2011 Jan;141(1):48-58.
15. Albain KS, Swann RS, Rusch VW, Turrisi AT III, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009 Aug;374(9687):379-386.
16. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004 Jan;350(4):351-360.
17. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006 Sep;7(9):719-727.
18. Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW; American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007; 132(3 Suppl):202S-265S.
|