| |
Abstract
Objectives: To describe the demographic characteristics and clinical presentation of Omani children with type 1 diabetes mellitus at Sultan Qaboos University Hospital, Muscat, Oman.
Methods: A retrospective analysis of all children with type 1 diabetes mellitus attending the Pediatric Endocrine Unit at Sultan Qaboos University Hospital, Oman from June 2006 to May 2013.
Results: One hundred and forty-four patients were included in the study. The mean±SD of age at diagnosis was 6.7 ± 3.7 years. The median duration of symptoms was 10 days (IQR; 5-14). The most commonly reported presenting symptoms were polyuria (94%), polydipsia (82%), and weight loss (59%). Diabetic ketoacidosis at initial presentation was diagnosed in 31% of the patients. Different insulin regimens were prescribed: multiple daily injections in 109 (76%) patients, twice daily insulin regimen in 23 (16%) patients, and insulin pump therapy in 12 (8%) patients. Family history of type 1 diabetes mellitus was present in 31 (22%) patients. There were no significant differences in presenting complaints (polyuria, p=0.182; polydipsia, p=0.848), duration of symptoms (p=0.331), reported weight loss (p=0.753), or diabetic ketoacidosis at presentation (p=0.608) between patients with and without family history of type 1 diabetes mellitus.
Conclusion: Polyuria, polydipsia and weight loss are the most common presenting symptoms. Family history of type 1 diabetes mellitus is highly prevalent among the studied patients. Diabetic ketoacidosis was found to be less common in Oman compared to other diabetes centers in the Middle East.
Keywords: Type 1 diabetes mellitus (T1DM); Children; Family history; Diabetic ketoacidosis (DKA); Insulin; Oman.
Introduction
Diabetes mellitus (DM) is a major public health problem. Nearly 65,000 children under 15 years of age develop type 1 diabetes mellitus (T1DM), increasing at a rate of 3% per year.1,2 With the global increase in the incidence and prevalence of diabetes, the number is predicted to reach 285 million cases in 2025.2 The worldwide incidence and prevalence of T1DM is quite variable, with highest incidence in Finland and Italy (36.5 and 36.8/100,000 per year, respectively).2,3 In Oman, the only available report on the incidence of childhood T1DM was in 1996 with documented incidence of 2.45 and 2.62/100,000 per year in 1993 and 1994, respectively.4 On the other hand, recent reports from other Gulf countries have documented higher incidence rates of T1DM in children as 15.4/100,000 per year in Kuwait,5 and 27.5/100,000 per year in Saudi Arabia.s.6
The classic presentations of T1DM in children are polyuria, polydipsia, and weight loss. A significant number of patients may present with diabetic ketoacidosis (DKA) which carries a significant risk of mortality to these children. Associated autoimmune diseases with T1DM mainly include celiac and thyroid diseases. The prevalence of biopsy proven celiac disease in Omani children with T1DM was documented to be 5.5%.7
There is paucity of published data on clinical characteristics of T1DM in Oman and Middle East. The aim of this study was to determine the demographic and clinical characteristics of T1DM in Omani children, and to compare them with other countries.
Methods
A retrospective analysis of children presented or referred to the Pediatric Endocrinology Unit at Sultan Qaboos University Hospital (SQUH), Oman, who were diagnosed to have T1DM during a period of seven years from June 2006-May 2013 were included in the study. Diagnosis of T1DM is usually made based on the World Health Organization criteria. The study included all children below 14 years of age who presented to the unit. Patients with diabetes associated syndromes such as Wolcott–Rallison syndrome, DIDMOAD syndrome (Diabetes Insipidus, Diabetes Mellitus, Optic Atrophy, and Deafness), type 2 diabetes mellitus or other causes including cystic fibrosis related diabetes (CFRD), steroid-induced diabetes and lipodystrophy were excluded. The study was approved by the Research and Ethics Committees, College of Medicine and Health Sciences, Sultan Qaboos University. The hospital records of these children were reviewed and missing data were completed by contacting the patients’ parents. Data collection included demographic, clinical and laboratory details. The clinical history at presentation was retrieved from electronic patient records. The data included history of polyuria, polydipsia, weight loss, and the duration of these symptoms, family history of T1DM, presence of DKA at initial presentation and current insulin therapy. DKA was defined as hyperglycemia above 11 mmol/L, arterial blood pH<7.30 or bicarbonate < 15mmol/L in the presence of ketonuria.8 The data on current insulin therapy included different regimens: twice daily insulin (intermediate acting with short acting insulin), multiple daily injections (Glargine® insulin once daily with pre-meal short acting insulin three times per day) and insulin pump therapy.
For statistical analysis, the data were analyzed using Statistical Package for Social Sciences program (SPSS, Version 20, IBM, Chicago, Illinois, USA) software. The mean (± standard deviation), or median (interquartile range IQR) were calculated for data as appropriate. Differences between patients with or without family history of T1DM in first degree relatives were assessed using chi-square test for categorical variables, and Mann-Whitney test for numerical variables. The differences between patients with or without DKA at presentation were assessed in the same manner. A p-value of <0.05 was used as a cut-off for all tests of statistical significance.
Results
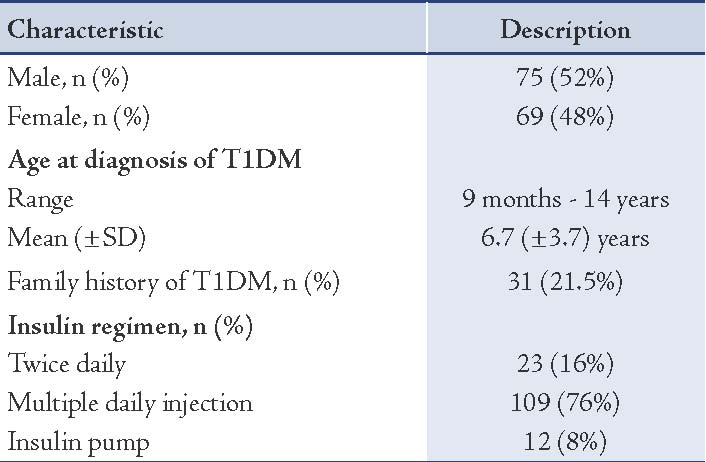
One hundred and forty-six patients were diagnosed as T1DM at SQUH during the 7-year study period from June 2006 to May 2013. Two patients were lost for follow-up. The demographic data of the remaining 144 patients is shown in Table 1. There were 75 (52%) males and 69 (48%) females. The overall mean age ± SD at diagnosis was 6.7 ± 3.7 years (range: 9 months to 14 years). Different insulin regimens were used that included multiple daily injections in 109 (76%) patients, twice daily insulin in 23 (16%) patients, and insulin pump in 12 (8%) patients.
Table 1: Demographic data of patients with type 1 diabetes mellitus (T1DM), n=144.
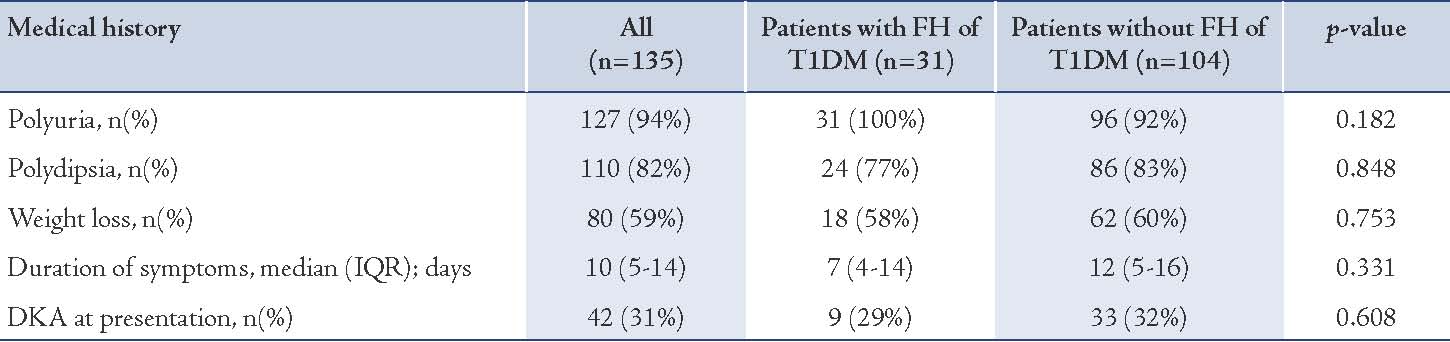
The medical history of the patients was retrieved (Table 2). Polyuria and polydipsia were the most frequent symptoms observed among the participants. History of polyuria and polydipsia were documented in 127 (94%) patients and 110 (82%) patients, respectively. Weight loss was noted by parents in 80 (59%) patients. The median duration of these symptoms before presentation to the hospital was 10 (IQR 5-14) days, with a range of 1-120 days. Forty-two patients (31%) had DKA at the time of first presentation to the hospital. Abdominal pain, vomiting and lethargy were the main complaints in those with documented DKA.
Family history of T1DM was present in 31 (22%) patients, of whom 22 patients had family history in first-degree relatives and 7 patients had family history in second-degree relatives. As illustrated in Table 2, there were no significant differences in history of polyuria (p=0.182), polydipsia (p=0.848), weight loss (p=0.753) or duration of these symptoms (p=0.331), between patients with or without family history of T1DM. Although the duration of symptoms was slightly less in cases with family history of T1DM (median: 7days, IQR: 4-14 days) vs. no family history of T1DM (median: 12 days, IQR: 5-16 days), this was statistically insignificant. In addition, there was no significant difference in the number of patients diagnosed with DKA at initial presentation between the two groups. Nine out of 31 patients with family history of T1DM (29%) had DKA at initial presentation compared to 33 out of 104 patients (32%) in those with no family history (p=0.608).
Table 2: Medical history in patients with T1DM stratified by family history (FH).
Discussion
The majority of the study participants presented with history of polyuria, polydipsia and weight loss. These symptoms can be recognized by the parents; therefore, awareness is crucial to avoid complications. The duration of symptoms found in the present cohort was less compared to those reported from other studies.9 These findings are consistent with other reported studies worldwide.9,10 The presence of DKA at initial presentation in the current cohort is comparatively higher than that reported from Sweden (12.8%), Finland (19%) and UK (25%).11-13
Nevertheless, our reported DKA incidence is much less compared to reports from Kuwait (37%) and the United Arab Emirates (80%).14,15 In a study from Al Medina in Saudi Arabia, DKA at presentation was present in 55% of the patients, and it was concluded that the presentation of type 1 diabetes in children from Saudi Arabia seems to be more severe than what is reported from developed countries.16 It is worth mentioning that the frequency of DKA at presentation of T1DM in children included in this current study is less compared to an earlier report at another center in Oman that documented an incidence of 41.7%.17 These findings could be explained by the progressive development of healthcare facilities in Oman, with prompt diagnosis and referral before development of DKA. This may also includes the increased awareness and self-education of the patients and families towards identifying the symptoms of diabetes and detection of the disease. Nevertheless, a larger prospective multicenter national study to document the overall incidence of DKA of newly diagnosed T1DM may consolidate these findings.
On the other hand, the higher frequency of DKA at presentation as compared to Finland, Germany, and UK might be due to different racial and environmental factors,16,18 as well as genetic heterogeneity between Asian and Caucasian populations.4 The median duration of symptoms in this study was 10 days, which is less than that documented in other reports,16 however, some patients presented with symptom duration for as long as 4 months, which may have serious implications on the child's health. Family history is an important risk factor for development of T1DM.19 Significant family history of T1DM was found in the current study which is less than that reported from Libya and Saudi Arabia.6,20 Although children with family history of T1DM in previous studies had less severe presentation,18 this was not documented in patients included in this study. This could represent an element of denial in the families with affected children who might try to avoid the fact of having another affected child. The majority of children included in this study received multiple daily injections which is internationally recommend for better metabolic control with less complications.21
The limitation of this study is the fact that this being a retrospective study from one centre in Oman, it may not represent all T1DM pediatric patients in the country. Consequently, the overall incidence of T1DM in Omani children could not be fully described.
Conclusion
T1DM has common symptoms which can be recognized by parents and physicians in order to improve the diagnosis of this serious disease and avoid its complications. Family history of T1DM was highly prevalent in this study which might be related to genetic factors. The current study results show that DKA at presentation is less prevalent compared to other Middle Eastern countries. It is recommended that a prospective multicenter study involving all the centers that care for diabetic patients in Oman be done to document the current incidence and prevalence of T1DM, different factors associated with the duration and severity of presentation with special emphasis on environmental, racial, cultural and genetic factors that may play a role.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J; Diabetes Mondiale (DIAMOND) Project Group. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000 Oct;23(10):1516-1526.
2. DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med 2006 Aug;23(8):857-866.
3. EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000 Mar;355(9207):873-876.
4. Soliman AT, al-Salmi IS, Asfour MG. Epidemiology of childhood insulin-dependent diabetes mellitus in the Sultanate of Oman. Diabet Med 1996 Jun;13(6):582-586.
5. Shaltout AA, Qabazard MA, Abdella NA, LaPorte RE, al Arouj M, Ben Nekhi A, et al; Kuwait Study Group of Diabetes in Childhood. High incidence of childhood-onset IDDM in Kuwait. Diabetes Care 1995 Jul;18(7):923-927.
6. Abduljabbar MA, Aljubeh JM, Amalraj A, Cherian MP. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi Med J 2010 Apr;31(4):413-418.
7. Al-Sinani S, Sharef SW, Al-Yaarubi S, Al-Zakwani I, Al-Naamani K, Al-Hajri A, et al. Prevalence of celiac disease in omani children with type 1 diabetes mellitus: a cross sectional study. Oman Med J 2013 Jul;28(4):260-263.
8. Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee W, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes 2009 Sep;10(Suppl 12):118-133.
9. Neu A, Ehehalt S, Willasch A, Kehrer M, Hub R, Ranke MB. Varying clinical presentations at onset of type 1 diabetes mellitus in children–epidemiological evidence for different subtypes of the disease? Pediatr Diabetes 2001 Dec;2(4):147-153.
10. Xin Y, Yang M, Chen XJ, Tong YJ, Zhang LH. Clinical features at the onset of childhood type 1 diabetes mellitus in Shenyang, China. J Paediatr Child Health 2010 Apr;46(4):171-175.
11. Samuelsson U, Stenhammar L. Clinical characteristics at onset of Type 1 diabetes in children diagnosed between 1977 and 2001 in the south-east region of Sweden. Diabetes Res Clin Pract 2005 Apr;68(1):49-55.
12. Hekkala A, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care 2007 Apr;30(4):861-866.
13. Ali K, Harnden A, Edge JA. Type 1 diabetes in children. BMJ 2011;342:d294.
14. Blanc N, Lucidarme N, Tubiana-Rufi N. [Factors associated with childhood diabetes manifesting as ketoacidosis and its severity]. Arch Pediatr 2003 Apr;10(4):320-325.
15. Punnose J, Agarwal MM, El Khadir A, Devadas K, Mugamer IT. Childhood and adolescent diabetes mellitus in Arabs residing in the United Arab Emirates. Diabetes Res Clin Pract 2002 Jan;55(1):29-33.
16. Al-Magamsi MS, Habib HS. Clinical presentation of childhood type 1 diabetes mellitus in the Al-Madina region of Saudi Arabia. Pediatr Diabetes 2004 Jun;5(2):95-98.
17. Soliman AT, al Salmi I, Asfour M. Mode of presentation and progress of childhood diabetes mellitus in the Sultanate of Oman. J Trop Pediatr 1997 Jun;43(3):128-132.
18. Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 2012 Nov;55(11):2878-2894.
19. Sipetić S, Vlajinac H, Kocev N, Marinković J, Radmanović S, Denić L. Family history and risk of type 1 diabetes mellitus. Acta Diabetol 2002 Sep;39(3):111-115.
20. Kadiki OA, Gerryo SE, Khan MM. Childhood diabetes mellitus in Benghazi (Libya). J Trop Pediatr 1987 Jun;33(3):136-139.
21. Al-Agha A, Ocheltree A, Hakeem A. Metabolic control in children and adolescents with insulin-dependent diabetes mellitus at King Abdul-Aziz University Hospital. J Clin Res Pediatr Endocrinol 2011;3(4):202-207.
|