|
Abstract
Objectives: To assess urinary NAG/ urinary creatinine (NAG/ Cr) ratio in patients with urological abnormalities (normal and abnormal ultrasonography) and compare it with normal healthy children.
Methods: A prospective study was conducted from November 2012 to April 2013. Urine samples were collected from 70 patients with various urological abnormalities, and from 40 healthy control groups, their age range (1 month-13 years). Children who were admitted to Emergency Pediatric department, Children Welfare Teaching Hospital / Medical City Complex, Baghdad, Iraq. The Glomerular filtration rate was in normal range in all of them. Urine samples were tested for NAG by (ELISA, Cusabio, China) while, both Serum creatinine (S-Cr) and urine creatinine (U-Cr) were estimated by Jaffe’s kinetic method.
Results: In this study 29% of the patients had normal ultrasonography and 72% abnormal ultrasonography. The current results revealed that NAG/creatinine index was significantly higher in all patients with (vesicoureteral reflux, hydronephrosis and pyelonephritis) in comparison with cystitis.
Conclusion: The assessment of urinary NAG could be considered as a useful marker in prediction of the (vesicoureteral reflux, hydronephrosis) .Urinary NAG is elevated in children with pyelonephritis and it can be considered as a further criterion in the diagnosis of upper urinary tract infection.
Keywords: Urinary N-acetyl-beta-D-glucosaminidase; urinary tract infection; vesicouretric reflux; hydronephrosis.
Introduction
The kidney plays a major role in maintaining constant volume and composition of the extracellular fluid. In this aspect, the kidney performs three basic functions: glomerular filtration, tubular reabsorption, and tubular secretion .The kidney function can be evaluated by a number of methods, including the assessment of urinary enzymes. Enzyme activity is normally low in urine and may increase when renal tubular cells are injured.1 The traditional laboratory approach for detection of renal disease does not allow for early detection of acute renal injury. Damage to renal tubules can be insufficient to result in a change in a parameter of kidney function such as serum creatinine. In addition, in cases of more extensive tubular injury, there is a lag in time between the injury and an increase in serum creatinine. Sensitive biological markers of renal tubular injury are needed in order to detect early kidney injury.2 Many clinical urological problems like hydronephrosis, vesicouretric reflux (VUR) and pyelonephritis carry a high risk, that require simple fast and noninvasive marker. Urinary problems, particularly VUR is potentially harmful because of the exposure of the kidney to the increased hydrodynamic pressure during voiding. Furthermore the incomplete emptying of the bladder and ureter on voiding predisposes the patient to urinary tract infection (UTI). This infection carries a relatively high risk of treatable urological problem and consequent development of renal scarring termed as reflux nephropathy.3 Vesicouretric reflux (VUR), is by far the most common abnormality seen in children,4 and cannot be diagnosed reliably by intravenous urography.5 Children, therefore require a voiding cystogram, if VUR is to be detected .This procedure entails placement of a catheter, and may require sedation in a toddlers, it also carries a high risk of radiation exposure to gonads.6 Dilatation of the renal pelvis and calyces, when combined with dilatation of the ureters known as hydroureteronephrosis. It may occur as a result of impairment of urine flow or the retrograde flow of urine. Hydronephrosis is the most common congenital condition that is detected by prenatal ultrasonography at an incidence of 1:100 to 1:500 by ultrasonographic studies. Approximately 10-20% patients with obstruction show progression of hydronephrosis or worsening renal functions.7,8 N-acetyl-β-D-glucosaminidase (NAG) is a lysosomal enzyme that is present in proximal tubular cells. The NAG has a relative high molecular weight of approximately 130000- 140000 daltons which does not permit its filtration through the glomerular basal membrane and it is rapidly cleared from the circulation by the liver. Thus, urinary NAG originates primarily from the proximal tubule, and increased urinary excretion is a consequence of renal tubular cell breakdown therefore, its urinary excretion is relatively constant with minimal diurnal changes. NAG is stable against changes in pH and temperature.9 The urinary NAG values should be expressed as a ratio to urinary creatinine concentration, as this relationship shows less variability than the urinary enzyme excretions related to volume or time.10
This study was done by the approval of biochemistry committee of Al-Nahrain University Medical College in partial Fulfillment of the requirements for the degree of Master of Science in Clinical Biochemistry.
Methods
A prospective study was conducted from November, 2012 to April, 2013 done. The study group included One hundred ten subjects, 70 patients were evaluated;(41 girls and 29 boys) Their age range between (1month-13years),classified as [cystitis (n=10), pyelonephritis (n=10), VUR (n=25), VUR with hydronephrosis (n=9) and hydronephrosis (n=16)], we enrolled children who were admitted to Emergency Pediatric department, Division of Pediatric Nephrology, Children Welfare Teaching Hospital /Medical City Complex, Baghdad, Iraq. Exclusion criteria included [chronic kidney diseases (CKD), Nephrotic syndrome, diabetes mellitus, Jaundice, and use of nephrotoxic drugs or Anti-epileptic agents (Carbamazepine (Tegretol) or Sodium Valproate (Depakin)]. Patients were divided into five main groups according to their clinical [fever, abdominal pain, anorexia and dysuria etc) and paraclinical findings [leukocyturia, positive urine culture, increased ESR (erythrocyte sedimentation rate), positive CRP(C- reactive protein) and ultrasonography, voiding cystourethrography.All patients had their kidney functions evaluated, While the apparently healthy control group consisted of 40 subjects (23 girls and 17 boys); their age ranged between (1month-13years), they were subdivided into three groups according to their age depending on the following references and studies11,12:
Control (1) group (n=11) - Less than 24 months (< 2 years).
Control (2) group (n=13) - Preschool age 24-60 months (2-5 years).
Control (3) group (n=16) - School age above 60 months (> 5 years).
Glomerular filtration rate (GFR) was in normal range in all of them using:
schwartz formula:
GFR (ml/min/1.73 m2) = K × Ht ÷ Pcr
(Where K = constant determined by regression analysis provided, Ht = height in cm Pcr = plasma creatinine).13,14
Urine and blood specimen were obtained from both patients and control group. A fresh random urine sample, collected at the first morning void was obtained on the admission time, all urine samples were analyzed by urinary dipstick and centrifuged to remove cellular components, the supernatants were stored at -20°C these urine samples were tested for N-acetyl-beta-D-glucosaminidase (NAG) (ELISA Kit Catalog No.CSB-E09450h .CUSABIO, China) .Both S-Cr and U-Cr were estimated by Jaffe’s kinetic method (Randox Laboratories, England).
The urinary NAG values were expressed as the urinary NAG/creatinine ratio. All of our patients were treated with same medication. The statistical analysis done by using social sciences software (SPSS version 17) Microsoft excel 2010 data were presented as mean ± SD for continuous data and percentage for categorical variables between every two groups were performed by student's t-test, while comparisons among groups were carried out by one-way analysis of variance (ANOVA). For all tests p-value <0.050 was considered statistically significant and <0.001 was considered highly significant. The receiver operating characteristic curve is used to define the diagnostic value (specificity and sensitivity) and best cutoff value of urinary NAG index.
Results
In this study, 70 patients were evaluated; 41 girls (59%) and 29 boys (41%). Their age range was within 1 month to 13 years with a mean age of 51.68 ± 40.76 months. While the apparently healthy control group in this study was 40 subjects, 23 girls (58%) and 17 boys (43%); their age ranged between 1 month-13 years with mean age 58.29 ± 42.13 months; 29% of the patients had normal ultrasonography and 72% abnormal ultrasonography. The current results revealed that the mean NAG/creatinine index was significantly higher in all patients than healthy control group at (p<0.001), histogram1 and table (1) shows the mean urinary NAG/creatinine index of all patients group, the highest mean level was observed among patients with Vesicoureteral Reflux (grade II, III, IV) (687.47 ± 176.1 U/g), while in hydronephrosis and pyelonephritis the mean levels were (398.18 ± 166.77 U/g) and (109.83 ± 37.81U/g) respectively then the level drop to normal in patients with cystitis (9.1 ± 4.4 U/g) at p value <0.001, and hence it demonstrates the differences between upper and lower UTI and the differences between normal and abnormal ultrasonography for selected diseases.
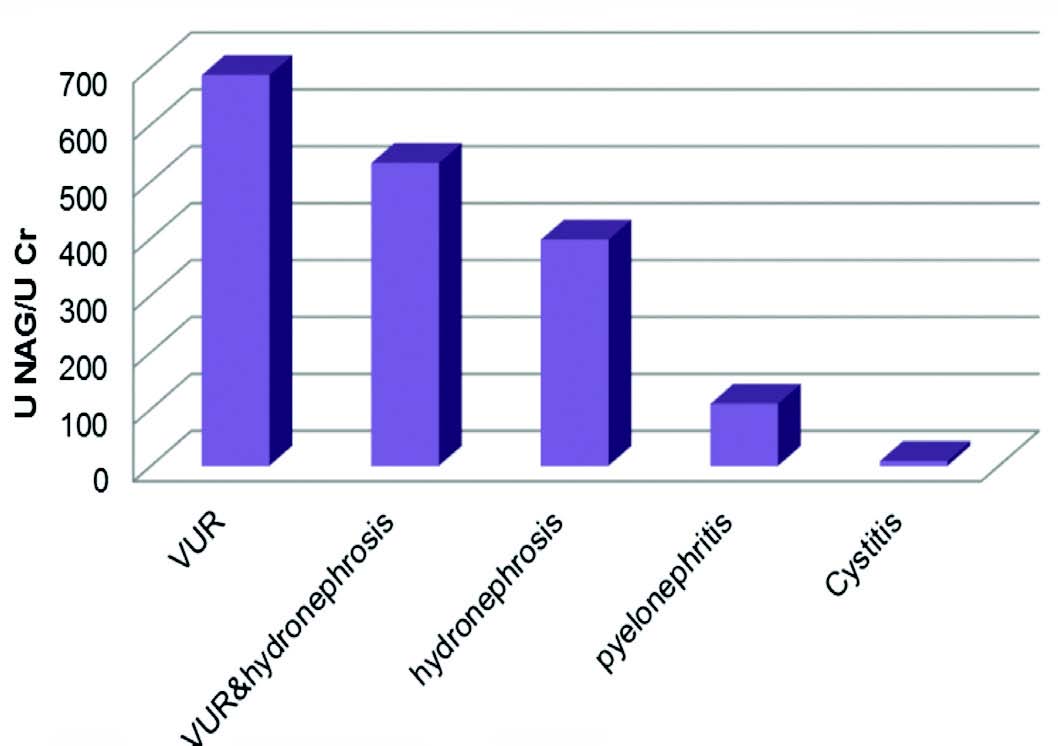
VUR: Vesicouretric reflux
Figure 1: Comparison U NAG/U Cr among patients groups.
Table 1: Comparison between all patients groups by ANOVA.
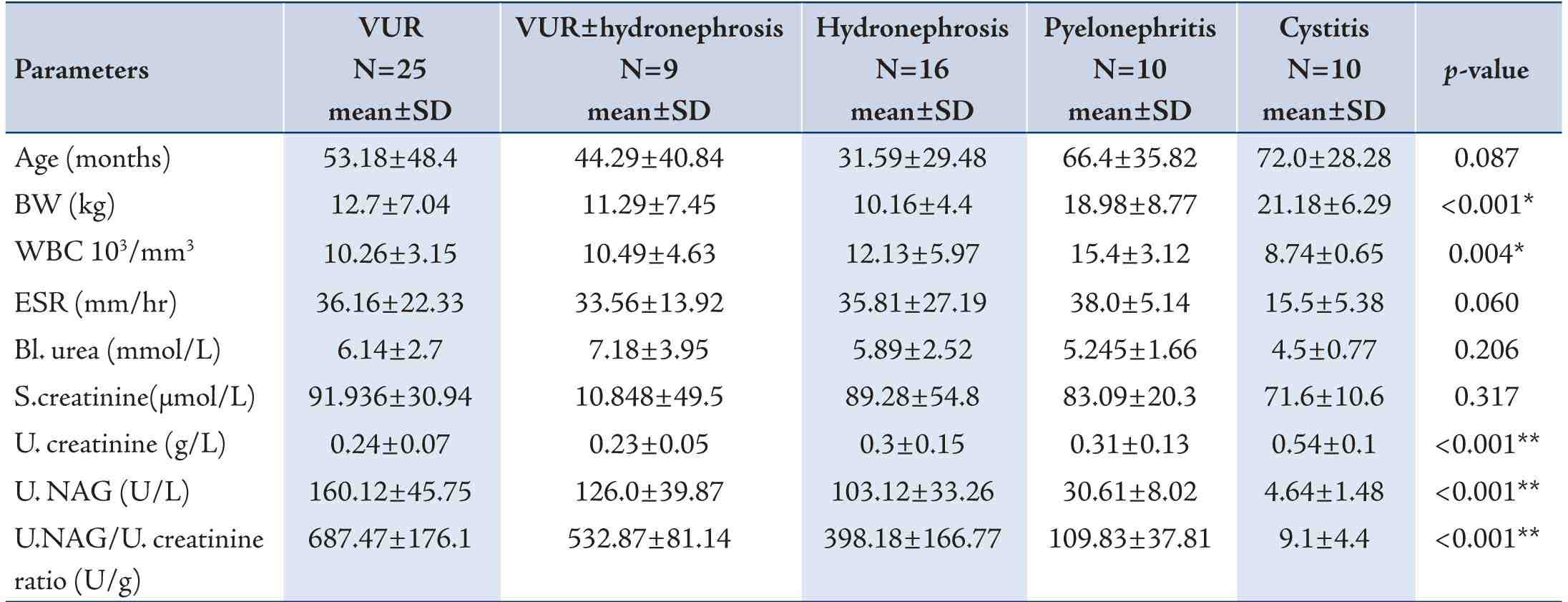
Table 2: Comparison between three control age groups by ANOVA test.
Table 3: Sensitivity and specificity of urine NAG/urine creatinine ratio in all patients groups except cystitis.
Table 4: Positive and negative predictive value of urine NAG/urine creatinine ratio.
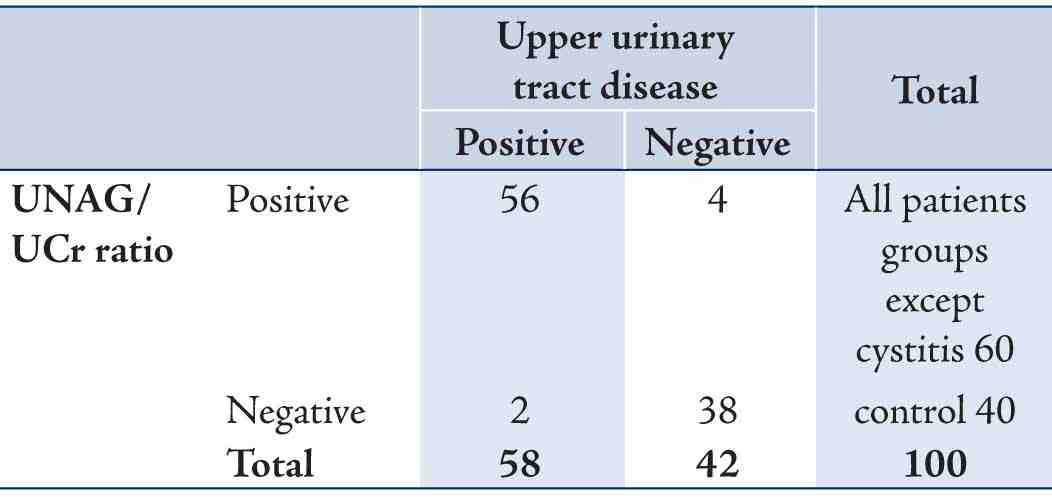
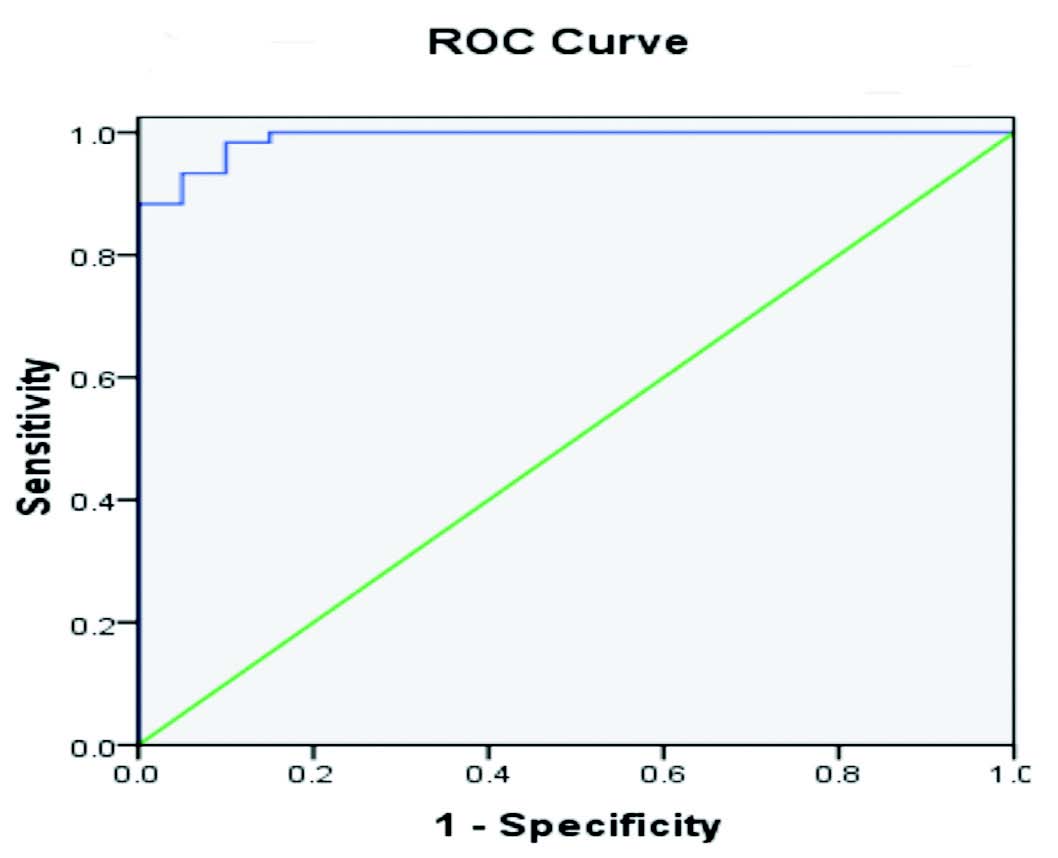
Figure 2: Receiver Operating Characteristic curve showing the diagnostic accuracy of Urinary NAG index (blue line).
By doing ANOVA test to compare the effect of age on NAG index, the results showed that the highest NAG index was in youngest age group [control (I), 67.16 ± 41.34U/g; control (2), 13.76 ± 5.96 U/g; control (3), 7.42 ± 3.12U/g at p<0.001] as shown in table 2.
Discussion
The use of biomarkers as a noninvasive tool in the diagnosis and management of various diseases is increasingly reported in the literature,15 also the use of these biomarkers is especially important in the pediatric age group as the use of invasive investigation is a matter of concern in children .Nevertheless, it is difficult to convince parents of the need for radiological investigation of their child after a UTI particularly in cystitis, but it is much easier if the patients have fever or hospitalized therefore, there is a need for noninvasive and sensitive test.16 Lee et al., in their review article have described the role of various biomarkers in pediatric urological diseases like vesicoureteral reflux (VUR) and pelvi-uretric junction obstruction (PUJO).17,18 The current study showed that there were no significant differences between males and females and this is in agreement with other studies conducted by,12,19 the urinary NAG/creatinine values were not gender dependent .In this study the measurement of NAG index of seventy patients admitted for treatment of recurrent UTI, the U-NAG/Cr index levels were significantly higher in VUR patients, hydronephrosis and Pyelonephritis compared to the control group at (p≤0.001), as shown in the table(1),while cystitis group had normal NAG index level, this is due to that the evidence of tubular dysfunction is common in children with vesicoureteral reflux,19 and as far as we are concerned, renal tubular damage that may occur due to hydronephrosis can be the etiologic factor for increased excretion of urinary proteins such as NAG. So increased level of urinary NAG seems to be due to the impact of severe hydronephrosis accompanying with cortical injury.19 Haung also reported that the level of urinary NAG was more elevated in patients with hydronephrosis than the control group,20 and in another Iranian study showed that the urinary NAG was elevated in children with pyelonephritis and it can be considered as a further criterion in the diagnosis of upper urinary tract infection.2 In the present study, the urinary NAG/creatinine values were highest in the youngest age groups (neonates and infants) Table 2, the excretion of NAG in normal individuals varies with age and shows strong age-dependence of the urinary NAG. This is might be due to high inter individual variability among neonate and infant age group or other possibility due to the fact that the urinary NAG/creatinine ratio tends to decrease with age in children, as a result of a concomitant rise in the urinary creatinine concentration.21
Conclusion
The assessment of urinary NAG could be considered as a useful marker in prediction of the VUR, hydronephrosis and renal tubular impairment in various disease states. Urinary NAG is elevated in children with pyelonephritis and it can be considered as a further criterion in the diagnosis of upper urinary tract infection.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
1. Chiu JS. Models used to assess renal function. Drug Dev Res 1994;32:247-255.
2. Mohkam M, Karimi A, Habibian S, Sharifian M. Urinary N-acetyl-beta-D-glucosaminidase as a diagnostic marker of acute pyelonephritis in children. Iran J Kidney Dis 2008 Jan;2(1):24-28.
3. Schwab CW Jr, Wu HY, Selman H, Smith GH, Snyder HM III, Canning DA. Spontaneous resolution of vesicoureteral reflux: a 15-year perspective. J Urol 2002 Dec;168(6):2594-2599.
4. Hellerstien S. Recurent urinary tract infections in children. Pediatr Infect Dis J 1982;1:271-281 .
5. Middleton AW jr.NixonGW.the lack of correlation between upper tract changes on excretory urography and vesicouretral reflex .j urol .1980; 123:227-228).
6. Seppänen U, Torniainen P, Kiviniitty K. Radiation gonad doses received by children in intravenous urography and micturition cysto-urethrography. Pediatr Radiol 1979 Jul;8(3):169-172.
7. Sidhu G, Beyene J, Rosenblum ND. Outcome of isolated antenatal hydronephrosis: a systematic review and meta-analysis. Pediatr Nephrol 2006 Feb;21(2):218-224.
8. Skalova S, Rejtar P, Kutilek S. Increased urinary N-acetyl-beta-D-glucosaminidase activity in children with hydronephrosis. Int Braz J Urol 2007 Jan-Feb;33(1):80-83, discussion 84-86.
9. Skálová S. The diagnostic role of urinary N-acetyl-beta-D-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Medica (Hradec Kralove) 2005;48(2):75-80.
10. Csáthy L, Oláh AV, Pócsi I, Varga J, Balla G, Price RG. [Age-dependent urinary N-acetyl-beta-D-glucosaminidase activity in healthy children]. Orv Hetil 1994 Jun;135(24):1301-1303.
11. Jack S. Elder. Urological disorders in infants and children. In: In: Nelson Textbook of Pediatrics, 19th ed. Robert M. Kliegman Bonita F. Stanston, Nina F Schor, Joseph W Geme, Richard E Behrman ed. Elsevier Saunders, Philadelphia 2011: p 1843.
12. Skalova.S and Chladek J. Urinary N-acetyl-b-D-glucosaminidase activity in healthy children, Nephro. j Hradec Králové, 2004 ;9,19-21 .
13. Lesley A. Stevens, Shani Shastri, Andrew S. Levey. Assessment of renal function. In: Jürgen Floege, Richard J. Johnson, John Feehally ed. Comprehensive clinical nephrology,4th ed. St.louis Elsevier Saunders. 2010: p 31-33.
14. Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987 Jun;34(3):571-590.
15. Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif 2010;29(4):357-365.
16. Fanos V, Mussap M, Osio D, Pizzini C, Plebani M. Urinary excretion of N-acetyl- beta-D-glucosaminidase and epidermal gfrowth factor in paediatric patients receiving cefixime prophylaxis for recurrent urinary tract infections. Clin Drug Investig 2001;21:511-518 .
17. Lee RS. Biomarkers for pediatric urological disease. Curr Opin Urol 2009 Jul;19(4):397-401.
18. Shokeir AA. Role of urinary biomarkers in the diagnosis of congenital upper urinary tract obstruction. Indian J Urol 2008 Jul;24(3):313-319.
19. Mohkam M, Karimi A, Sharifian M, Dalirani R, Habibian S, Jadali F. Is urinary N-acetyl-beta-D-glucosaminidase a marker of urological abnormality in children? MJIRI 2009;23:36-41.
20. Huang H, Chen J, Chen C. Circulating adhesion molecules and neutral endopeptidase enzymuria in patients with urolithiasis and hydronephrosis. Urology 2000 Jun;55(6):961-965.
21. Skinner AM, Addison GM, Price DA. Changes in the urinary excretion of creatinine, albumin and N-acetyl-beta-D-glucosaminidase with increasing age and maturity in healthy schoolchildren. Eur J Pediatr 1996 Jul;155(7):596-602.
|